K+-Driven Cl−/HCO3− Exchange Mediated by Slc4a8 and Slc4a10
Abstract
:1. Introduction
2. Results
2.1. Expression of Mouse Slc4a8 and Slc4a10 in CHO-K1 Cells
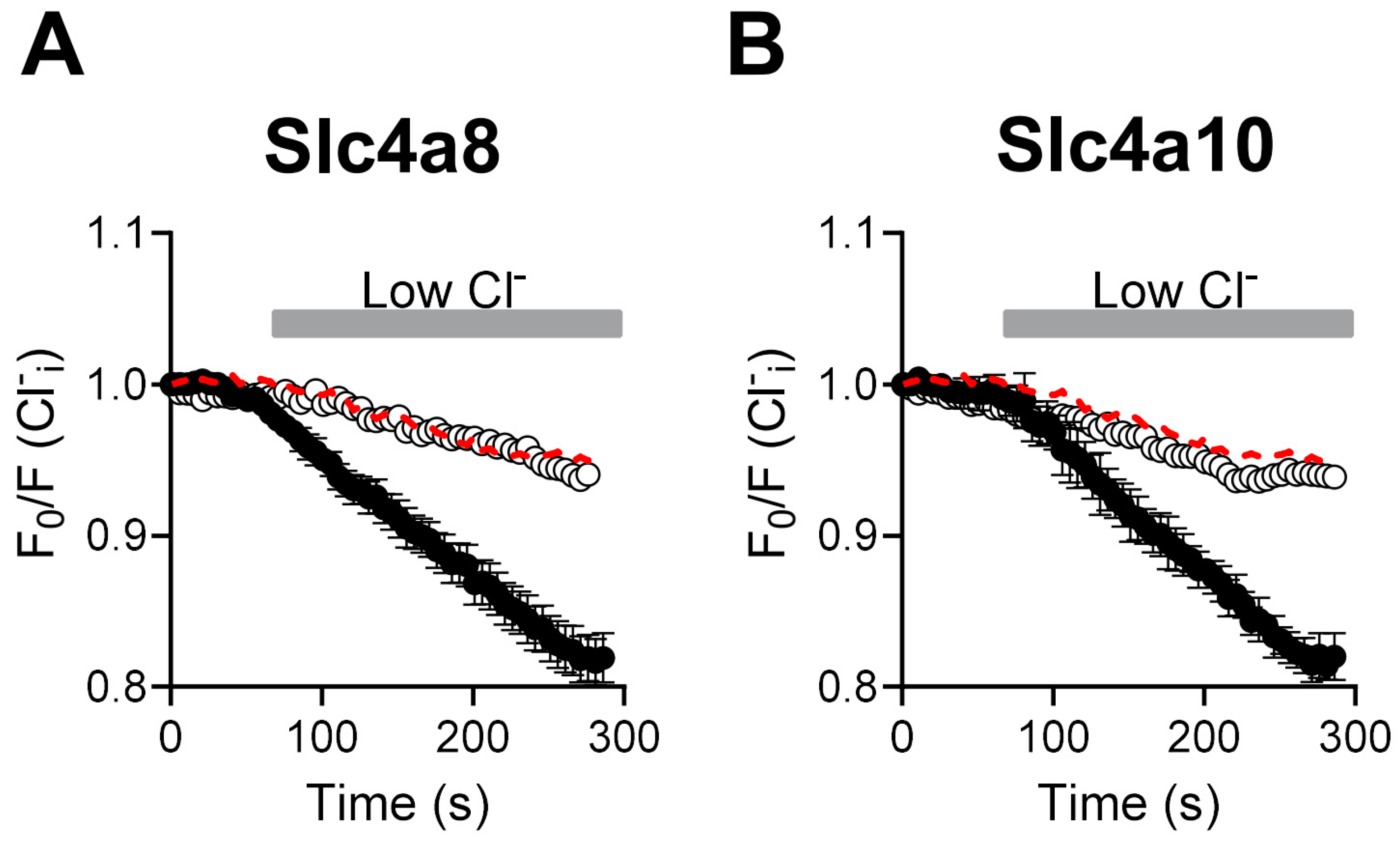
2.2. Cl− Fluxes Are Associated with Slc4a8 and Slc4a10 Expression in CHO-K1 Cells
2.3. Na+- and K+-Dependence of Slc4a8- and Slc4a10-Mediated Cl−/HCO3−-Exchange in CHO-K1 Cells
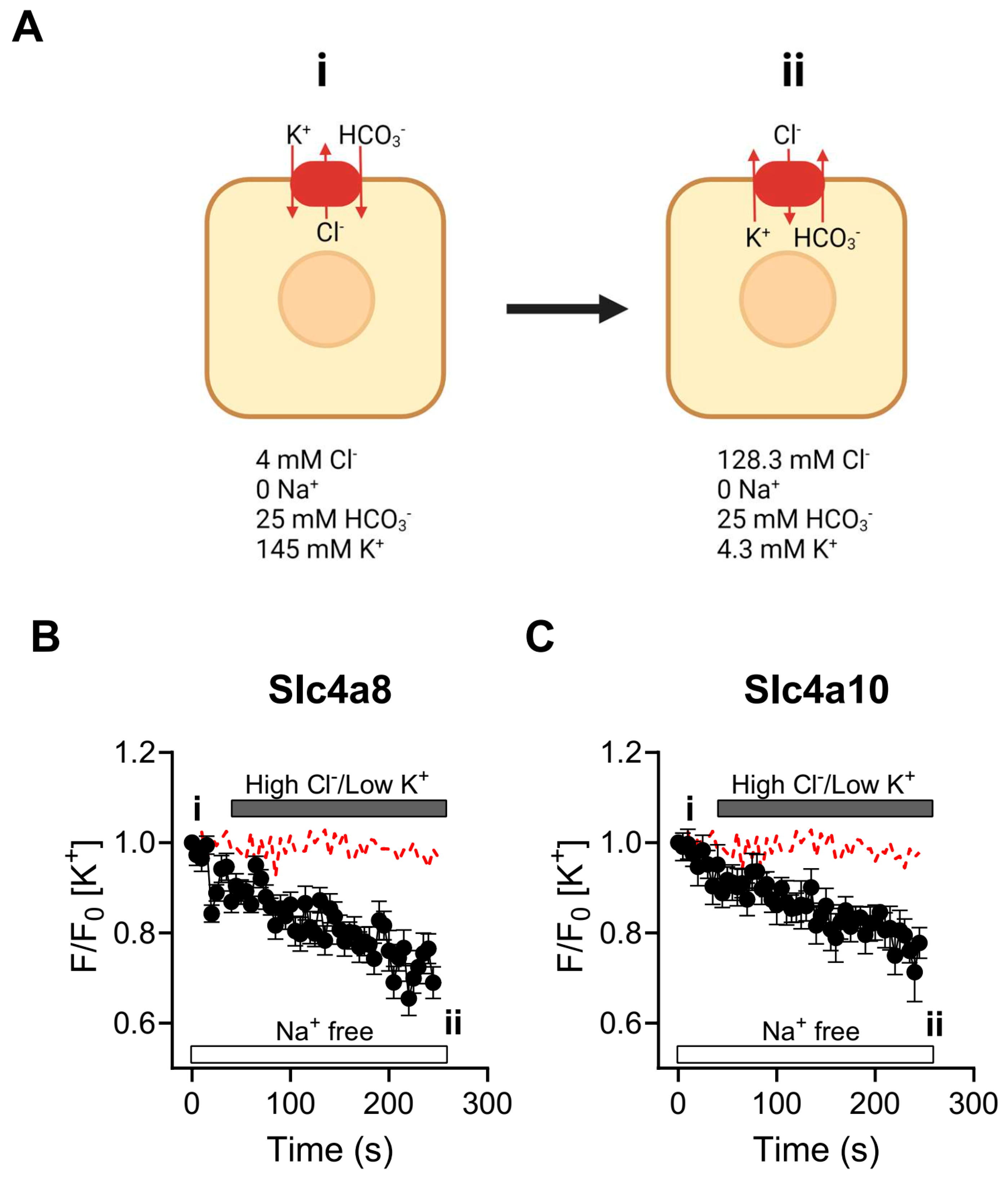
2.4. Slc4a8- and Slc4a10-Mediated K+ Transport in CHO-K1 Cells
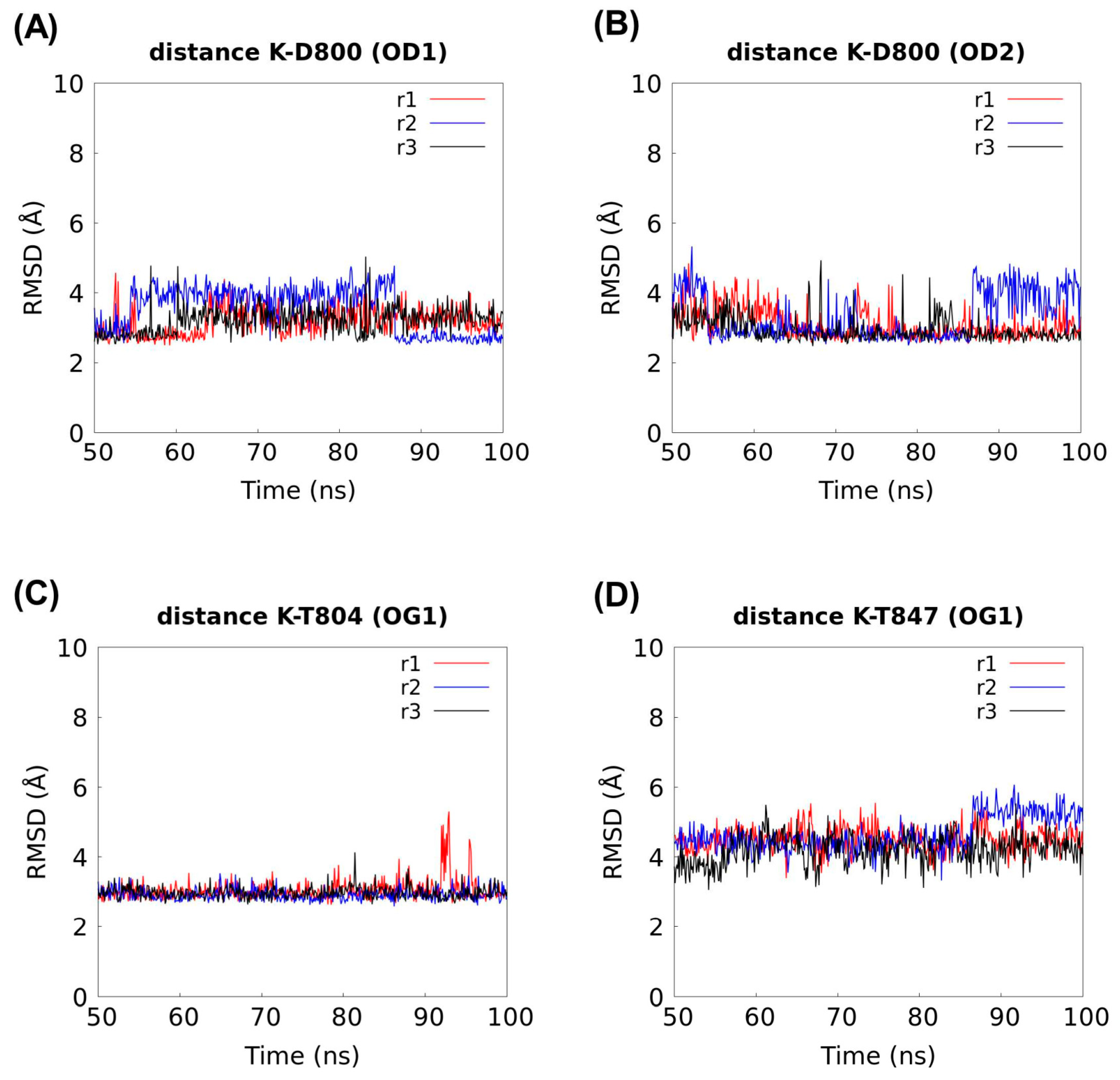
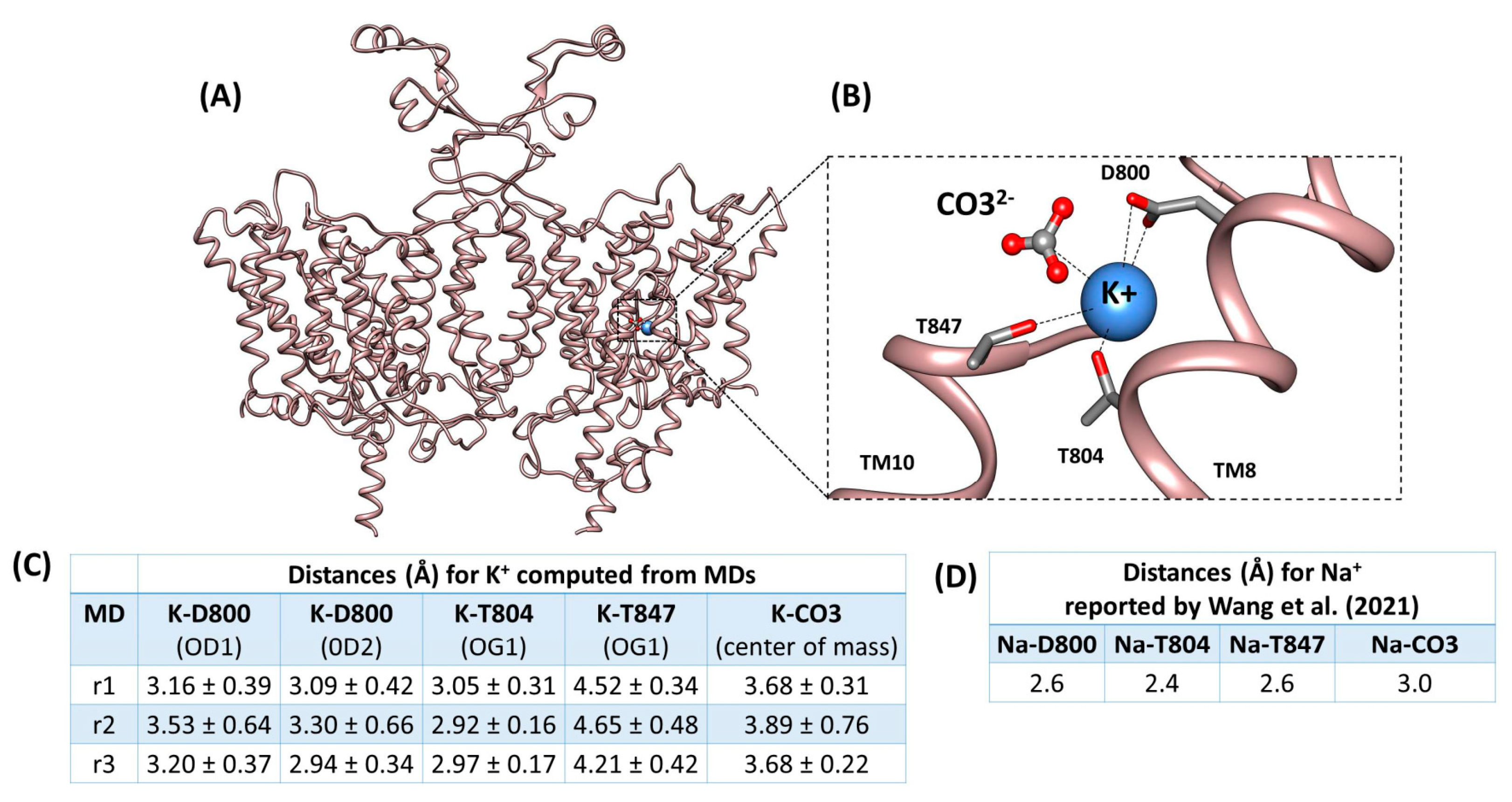
2.5. Molecular Dynamics Supports K+ Occupancy at the Na+ Coordination S1 Site in the Cryo-EM rSlc4a8 Structure
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boron, W.F.; McCormick, W.C.; Roos, A. pH regulation in barnacle muscle fibers: Dependence on extracellular sodium and bicarbonate. Am. J. Physiol. 1981, 240, C80–C89. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.M.; Boron, W.F. Role of choloride transport in regulation of intracellular pH. Nature 1976, 264, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Jalimarada, S.S.; Ogando, D.G.; Vithana, E.N.; Bonanno, J.A. Ion transport function of SLC4A11 in corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4330–4340. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.K.; Schneider, H.P.; Morgan, P.E.; Deitmer, J.W.; Casey, J.R. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am. J. Physiol. Cell Physiol. 2016, 311, C735–C748. [Google Scholar] [CrossRef] [PubMed]
- Ogando, D.G.; Jalimarada, S.S.; Zhang, W.; Vithana, E.N.; Bonanno, J.A. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am. J. Physiol. Cell Physiol. 2013, 305, C716–C727. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Li, Q.; Shcheynikov, N.; Zeng, W.; Muallem, S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol. Cell 2004, 16, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.F.; Chen, A.P.; Parker, M.D.; Boron, W.F. The SLC4 family of bicarbonate HCO3− transporters. Mol. Asp. Med. 2013, 34, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Almomani, E.Y.; Chu, C.Y.; Cordat, E. Mis-trafficking of bicarbonate transporters: Implications to human diseases. Biochem. Cell Biol. 2011, 89, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D. Mouse models of SLC4-linked disorders of HCO3−-transporter dysfunction. Am. J. Physiol. Cell Physiol. 2018, 314, C569–C588. [Google Scholar] [CrossRef]
- Pena-Munzenmayer, G.; Catalan, M.A.; Kondo, Y.; Jaramillo, Y.; Liu, F.; Shull, G.E.; Melvin, J.E. Ae4 (Slc4a9) Anion Exchanger Drives Cl− Uptake-dependent Fluid Secretion by Mouse Submandibular Gland Acinar Cells. J. Biol. Chem. 2015, 290, 10677–10688. [Google Scholar] [CrossRef]
- Pena-Munzenmayer, G.; George, A.T.; Shull, G.E.; Melvin, J.E.; Catalan, M.A. Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl−/HCO3− exchanger. J. Gen. Physiol. 2016, 147, 423–436. [Google Scholar] [CrossRef]
- Amlal, H.; Burnham, C.E.; Soleimani, M. Characterization of Na+/HCO3− cotransporter isoform NBC-3. Am. J. Physiol. 1999, 276, F903–F913. [Google Scholar]
- Damkier, H.H.; Aalkjaer, C.; Praetorius, J. Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J. Biol. Chem. 2010, 285, 26998–27007. [Google Scholar] [CrossRef]
- Grichtchenko, I.I.; Choi, I.; Zhong, X.; Bray-Ward, P.; Russell, J.M.; Boron, W.F. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl−-HCO3− exchanger. J Biol Chem 2001, 276, 8358–8363. [Google Scholar] [CrossRef]
- Parker, M.D.; Musa-Aziz, R.; Rojas, J.D.; Choi, I.; Daly, C.M.; Boron, W.F. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J. Biol. Chem. 2008, 283, 12777–12788. [Google Scholar] [CrossRef]
- Minta, A.; Tsien, R.Y. Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 1989, 264, 19449–19457. [Google Scholar] [CrossRef]
- Wang, W.; Tsirulnikov, K.; Zhekova, H.R.; Kayık, G.; Khan, H.M.; Azimov, R.; Abuladze, N.; Kao, L.; Newman, D.; Noskov, S.Y.; et al. Cryo-EM structure of the sodium-driven chloride/bicarbonate exchanger NDCBE. Nat. Commun. 2021, 12, 5690. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yano, H.; Nagashima, K.; Seino, S. The Na+-driven Cl−/HCO3− exchanger. Cloning, tissue distribution, and functional characterization. J. Biol. Chem. 2000, 275, 35486–35490. [Google Scholar] [CrossRef]
- Sinning, A.; Liebmann, L.; Kougioumtzes, A.; Westermann, M.; Bruehl, C.; Hübner, C.A. Synaptic glutamate release is modulated by the Na+-driven Cl−/HCO₃− exchanger Slc4a8. J. Neurosci. 2011, 31, 7300–7311. [Google Scholar] [CrossRef]
- Fasham, J.; Huebner, A.K.; Liebmann, L.; Khalaf-Nazzal, R.; Maroofian, R.; Kryeziu, N.; Wortmann, S.B.; Leslie, J.S.; Ubeyratna, N.; Mancini, G.M.S.; et al. SLC4A10 mutation causes a neurological disorder associated with impaired GABAergic transmission. Brain 2023, 146, 4547–4561. [Google Scholar] [CrossRef]
- Maroofian, R.; Zamani, M.; Kaiyrzhanov, R.; Liebmann, L.; Karimiani, E.G.; Vona, B.; Huebner, A.K.; Calame, D.G.; Misra, V.K.; Sadeghian, S.; et al. Biallelic variants in SLC4A10 encoding a sodium-dependent bicarbonate transporter lead to a neurodevelopmental disorder. Genet. Med. 2023, 26, 101034. [Google Scholar] [CrossRef]
- Virkki, L.V.; Choi, I.; Davis, B.A.; Boron, W.F. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am. J. Physiol. Cell Physiol. 2003, 285, C771–C780. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Rink, T.J.; Tsien, R.Y.; Pozzan, T. Cytoplasmic pH and free Mg2+ in lymphocytes. J. Cell Biol. 1982, 95, 189–196. [Google Scholar] [CrossRef]
- Illsley, N.P.; Verkman, A.S. Membrane chloride transport measured using a chloride-sensitive fluorescent probe. Biochemistry 1987, 26, 1215–1219. [Google Scholar] [CrossRef]
- Chao, A.C.; Dix, J.A.; Sellers, M.C.; Verkman, A.S. Fluorescence measurement of chloride transport in monolayer cultured cells. Mechanisms of chloride transport in fibroblasts. Biophys. J. 1989, 56, 1071–1081. [Google Scholar] [CrossRef]
- Edelstein, A.; Amodaj, N.; Hoover, K.; Vale, R.; Stuurman, N. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 2010, 92, 14.20.1–14.20.17. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing (SC06), Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Merz, K.M. Application of the Nose-Hoover chain algorithm to the study of protein dynamics. J. Phys. Chem. 1996, 100, 1927–1937. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]



| Component | Solution Name | ||||||
|---|---|---|---|---|---|---|---|
| (mM) | A | B | C | D | E | F | G |
| Na+ | 145 | 145 | 145 | 145 | |||
| K+ | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | 145 |
| NMDG | 120 | 120 | |||||
| Ca2+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mg2+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline | 25 | 25 | |||||
| Cl− | 128.3 | 4 | 128.3 | 4 | 153.3 | 4 | 4 |
| HCO3− | 25 | 25 | 25 | 25 | 25 | ||
| Gluconate | 124.3 | 149.3 | 120 | ||||
| Glutamate | 124.3 | ||||||
| Glucose | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| HEPES | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| pH | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 |
| Solution (mM) | Na+ 5 | Na+ 25 | Na+ 50 | Na+ 100 | Na+ 125 | Na+ 150 | K+ 5 | K+ 25 | K+ 50 | K+ 100 | K+ 125 | K+ 150 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | 5 | 25 | 50 | 100 | 125 | 150 | ||||||
| K+ | 5 | 25 | 50 | 100 | 125 | 150 | ||||||
| NMDG | 120 | 125 | 100 | 50 | 25 | 120 | 125 | 100 | 50 | 25 | ||
| Ca2+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mg2+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline | 25 | 25 | ||||||||||
| Cl− | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| HCO3− | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Glutamate | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| Glucose | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| HEPES | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| pH | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Münzenmayer, G.; George, A.T.; Llontop, N.; Mazola, Y.; Apablaza, N.; Spichiger, C.; Brauchi, S.; Sarmiento, J.; Zúñiga, L.; González, W.; et al. K+-Driven Cl−/HCO3− Exchange Mediated by Slc4a8 and Slc4a10. Int. J. Mol. Sci. 2024, 25, 4575. https://doi.org/10.3390/ijms25084575
Peña-Münzenmayer G, George AT, Llontop N, Mazola Y, Apablaza N, Spichiger C, Brauchi S, Sarmiento J, Zúñiga L, González W, et al. K+-Driven Cl−/HCO3− Exchange Mediated by Slc4a8 and Slc4a10. International Journal of Molecular Sciences. 2024; 25(8):4575. https://doi.org/10.3390/ijms25084575
Chicago/Turabian StylePeña-Münzenmayer, Gaspar, Alvin T. George, Nuria Llontop, Yuliet Mazola, Natalia Apablaza, Carlos Spichiger, Sebastián Brauchi, José Sarmiento, Leandro Zúñiga, Wendy González, and et al. 2024. "K+-Driven Cl−/HCO3− Exchange Mediated by Slc4a8 and Slc4a10" International Journal of Molecular Sciences 25, no. 8: 4575. https://doi.org/10.3390/ijms25084575






