The Role of the Placental Enzyme Indoleamine 2,3-Dioxygenase in Normal and Abnormal Human Pregnancy
Abstract
:1. Introduction
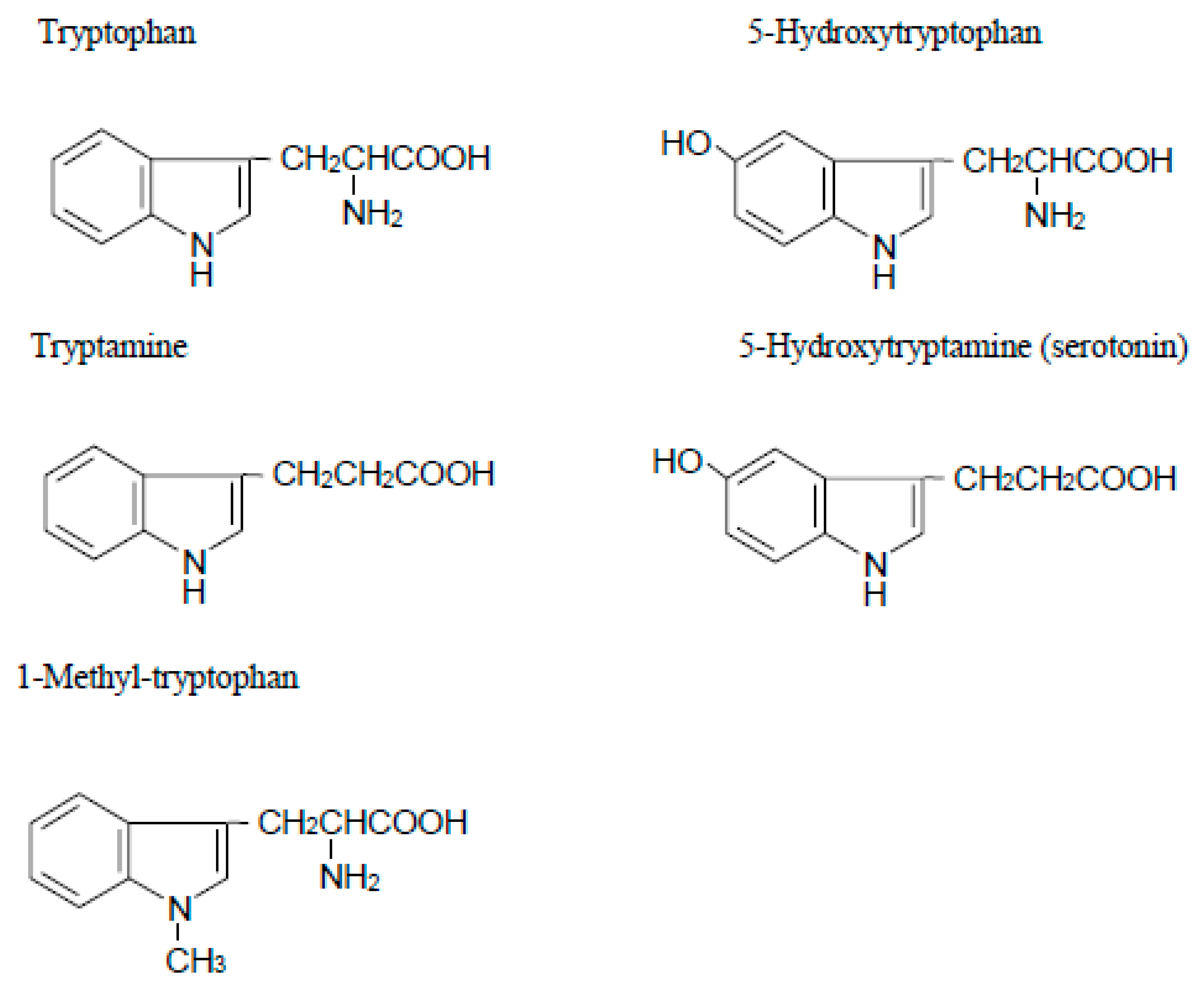
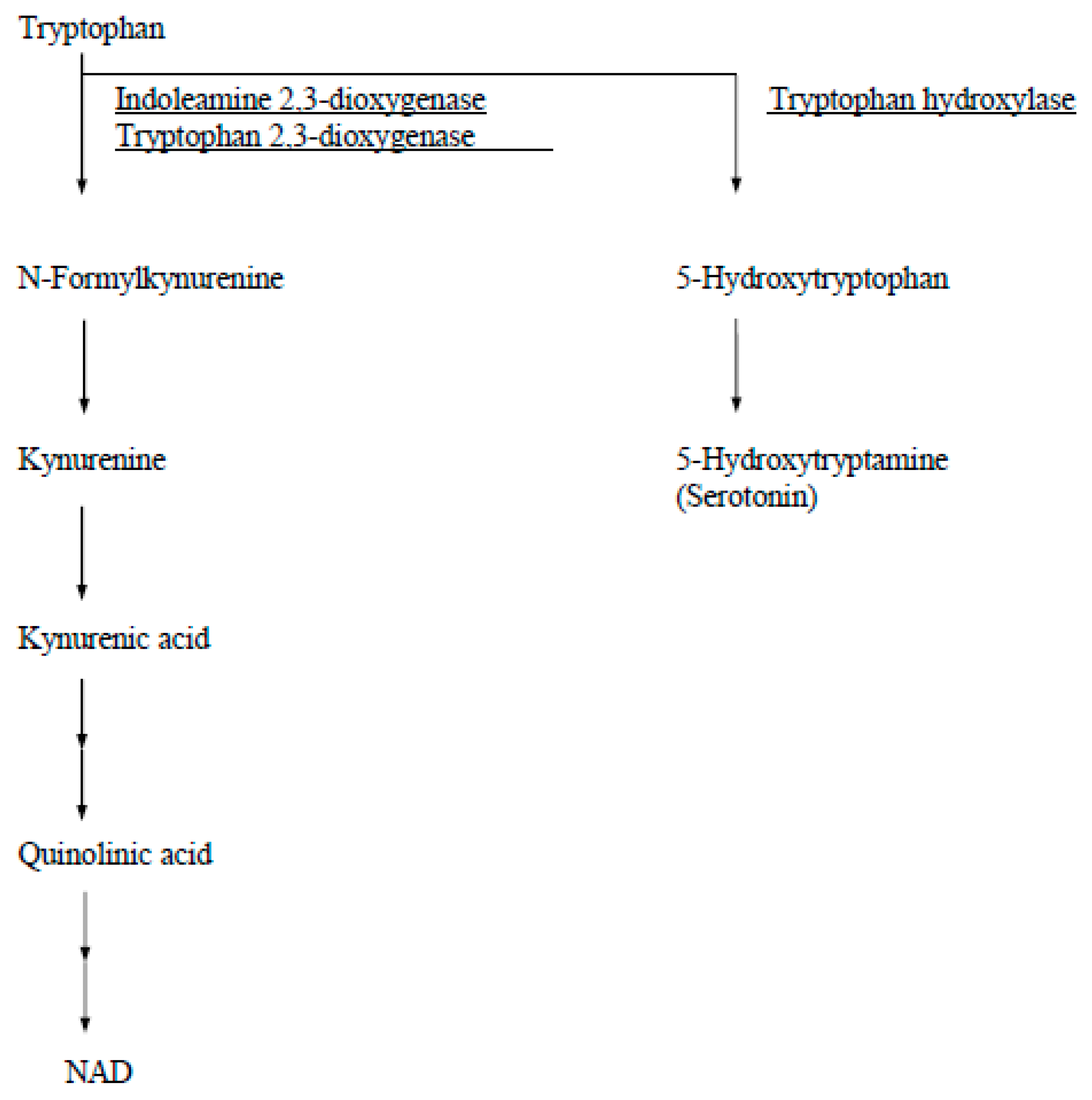
2. Indoleamine 2,3-Dioxygenase (IDO)
3. Paradox of Immunological Tolerance toward the Fetus during Pregnancy
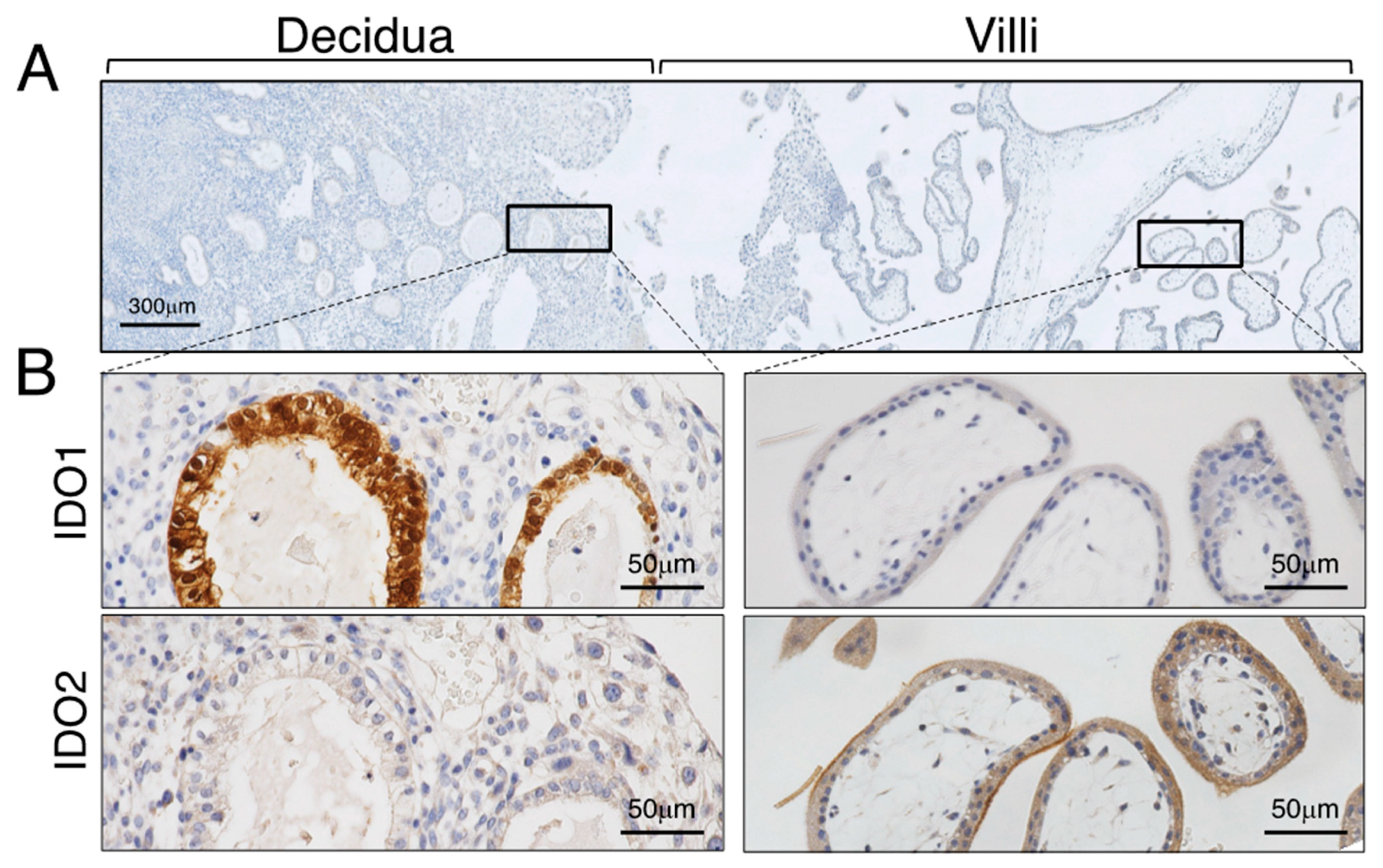
4. Localization and Characterization of Human Placental IDO
5. Tryptophan Catabolism by Placental IDO in Human Pregnancy: A Comparison of Normal Pregnancy and Pre-Eclampsia
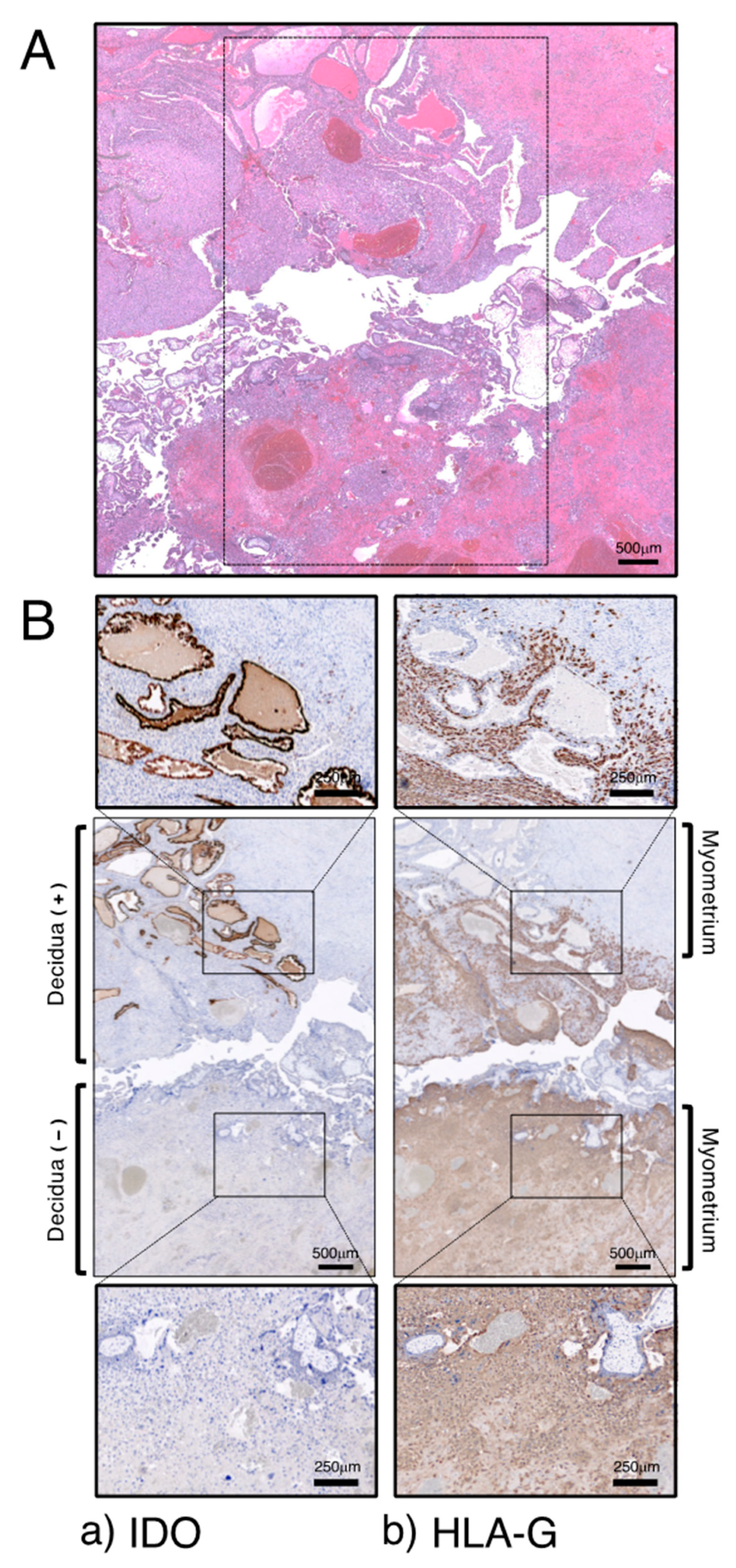
6. Placental IDO and Trophoblast Invasion: Implications for the Pathogenesis of PAS
7. Conclusions
Funding
Conflicts of Interest
References
- Yoshida, R.; Hayaishi, O. Indoleamine 2,3-dioxygenase. Methods Enzymol. 1987, 142, 188–195. [Google Scholar] [PubMed]
- Yamazaki, F.; Kuroiwa, T.; Takikawa, O.; Kido, R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem. J. 1985, 230, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A.R.; Spyropoulou, I.; Redman, C.W.G.; Takikawa, O.; Katsuki, T.; Hara, T.; Ohama, K.; Sargent, I. Indoleamine 2,3-dioxygenase: Distribution and function in the developing human placenta. J. Reprod. Immunol. 2004, 61, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A.R.; Sargent, I.L.; Redman, C.W.G. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J. Physiol. 2001, 535, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Anthony, J.; Davey, D.A.; Rees, A.; Tiltman, A.; Vercruysse, L.; Van Assche, A.N.D.R.E. Placental bed spiral arteries in the hypertensive dis-orders of pregnancy. Br. J. Obstet. Gynaecol. 1991, 98, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Davidge, S.T. Oxidative stress and altered endothelial cell function in preeclampsia. Semin. Reprod. Med. 1998, 16, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- von Dadelszen, P.; Wilkins, T.; Redman, C.W. Maternal peripheral blood leukocytes in normal and pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1999, 106, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultra-sound imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef]
- Jauniaux, E.; Burton, G.J. Placenta accreta spectrum: A need for more research on its aetiopathogenesis. BJOG 2018, 125, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Reister, F.; Frank, H.G.; Kingdom, J.C.; Heyl, W.; Kaufmann, P.; Rath, W.; Huppertz, B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Mod. Pathol. 2001, 81, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an im-munoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hirata, F.; Hayaishi, O. New degradative routes of 5-hydroxytryptophan and serotonin by intestinal tryptophan 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 1972, 47, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.S.; Pogson, C.I.; Smith, S.A. Indoleamine 2,3-dioxygenase. A new, rapid, sensitive radiometric assay and its application to the study of the enzyme in rat tissues. Biochem. J. 1980, 189, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Nukiwa, T.; Watanabe, Y.; Fujiwara, M.; Hirata, F.; Hayaishi, O. Regulation of indoleamine 2,3-dioxygenase activity in the small intestine and the epididymis of mice. Arch. Biochem. Biophys. 1980, 203, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Urade, Y.; Tokuda, M.; Hayaishi, O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc. Natl. Acad. Sci. USA 1979, 76, 4084–4086. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Pan, H.; Kwok, O.; Dubey, J.P. Human indoleamine 2,3-dioxygenase inhibits Toxoplasma gondii growth in fibroblast cells. J. Interferon Res. 1994, 14, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, O.; Habara Ohkubo, A.; Yoshida, R. Induction of indoleamine 2,3-dioxygenase in tumor cells transplanted into allogene-ic mouse: Interferon-gamma is the inducer. Adv. Exp. Med. Biol. 1991, 294, 437–444. [Google Scholar] [PubMed]
- Yoshida, R.; Park, S.W.; Yasui, H.; Takikawa, O. Tryptophan degradation in transplanted tumor cells undergoing rejection. J. Immunol. 1988, 141, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Gupta, S.L. Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem. Biophys. Res. Commun. 1990, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Sanchez-Perez, A.; Weiser, S.; Austin, C.J.D.; Astelbauer, F.; Miu, J.; McQuillan, J.A.; Stocker, R.; Jermiin, L.S.; Hunt, N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 2007, 396, 203–213. [Google Scholar] [CrossRef]
- Metz, R.; Duhadaway, J.B.; Kamasani, U.; Laury-Kleintop, L.; Muller, A.J.; Prendergast, G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007, 67, 7082–7087. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Yamamoto, Y.; Kawasoe, M.; Arioka, Y.; Murakami, Y.; Hoshi, M.; Saito, K. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: The absence of IDO1 upregulates IDO2 expression in the epididymis. J. Histochem. Cytochem. 2012, 60, 854–860. [Google Scholar] [CrossRef]
- Cady, S.G.; Sono, M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and be-ta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch. Biochem. Biophys. 1991, 291, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Sivakumar, J.; Chandler, P.; Smith, K.; Molina, H.; Mao, D.; Munn, D.H. Prevention of T cell–driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2001, 2, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Medawar, P.B. Some immunological and endocrinological problems raised by evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953, 7, 320–328. [Google Scholar]
- Bonney, E.A.; Matzinger, P. The maternal immune system’s interaction with circulating fetal cells. J. Immunol. 1997, 158, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.; Hammer, A.; Dohr, G.; Hunt, J.S. HLA Expression at the maternal-fetal interface. Dev. Immunol. 1998, 6, 197–204. [Google Scholar] [CrossRef]
- King, A.; Boocock, C.; Sharkey, A.M.; Gardner, L.; Beretta, A.; Siccardi, A.G.; Loke, Y.W. Evidence for the expression of HLAA-C class I mRNA and protein by human first trimester trophoblast. J. Immunol. 1996, 156, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Stites, D.P.; Siiteri, P.K. Steroids as Immunosuppressants in Pregnancy. Immunol. Rev. 1983, 75, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Paul, P.; Rouas Freiss, N.; Kirszenbaum, M.; Dausset, J.; Carosella, E.D. Molecular and im0munologic aspects of the non-classical HLA class I antigen HLA-G: Evidence for an important role in the maternal tolerance of the fetal allograft. Am. J. Reprod. Immunol. 1998, 40, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, A.M.; Schulte, H.M.; Thuneke, I.; Erdmann, I.; Bamberger, C.M.; Asa, S.L. Expression of the apoptosis-inducing Fas ligand (FasL) in human first and third trimester placenta and choriocarcinoma cells. J. Clin. Endocrinol. Metab. 1997, 82, 3173–3175. [Google Scholar] [CrossRef]
- Zorzi, W.; Thellin, O.; Coumans, B.; Melot, F.; Hennen, G.; Lakaye, B.; Igout, A.; Heinen, E. Demonstration of the expression of CD95 ligand transcript and protein in human placenta. Placenta 1998, 19, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Hara, T.; Katsuki, T.; Toyofuku, A.; Katsura, Y.; Takikawa, O.; Fujii, T.; Ohama, K. Mechanisms regulating the expression of indoleamine 2,3-dioxygenase during decidualization of human endometrium. Hum. Reprod. 2004, 19, 1222–1230. [Google Scholar] [CrossRef]
- Sedlmayr, P.; Blaschitz, A.; Wintersteiger, R.; Semlitsch, M.; Hammer, A.; MacKenzie, C.R.; Walcher, W.; Reich, O.; Takikawa, O.; Dohr, G. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol. Hum. Reprod. 2002, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, O.; Kuroiwa, T.; Yamazaki, F.; Kido, R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan deg-radation in its anticellular activity. J. Biol. Chem. 1988, 263, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Koh, I.; Sugimoto, J. Localization of Indoleamine 2,3-dioxygenase-1 and indoleamine 2,3-dioxygenase-2 at the human maternal-fetal interface. Int. J. Tryptophan Res. 2020, 13, 1178646920984163. [Google Scholar] [CrossRef] [PubMed]
- Ligam, P.; Manuelpillai, U.; Wallace, E.; Walker, D. Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: Implications for role of the kynurenine pathway in pregnancy. Placenta 2005, 26, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Santoso, D.; Rogers, P.; Wallace, E.; Manuelpillai, U.; Walker, D.; Subakir, S.B. Localization of indoleamine 2,3-dioxygenase and 4-hydroxynonenal in normal and pre-eclamptic placentae. Placenta 2002, 23, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Schrocksnadel, H.; Baier Bitterlich, G.; Dapunt, O.; Wachter, H. Activated cellular immunity and decreased serum trypto-phan in healthy pregnancy. Adv. Exp. Med. Biol. 1996, 398, 149–153. [Google Scholar] [PubMed]
- Schröcksnadel, H.; Baierbitterlich, G.; Dapunt, O.; Wachter, H.; Fuchs, D. Decreased plasma tryptophan in pregnancy. Obstet. Gynecol. 1996, 88, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A.R.; Sargent, I.L.; Redman, C.W.G. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am. J. Obstet. Gynecol. 2003, 188, 719–726. [Google Scholar] [CrossRef]
- Contursi, C.; Wang, I.M.; Gabriele, L.; Gadina, M.; O’Shea, J.; Morse, H.C., 3rd; Ozato, K. IFN consensus sequence binding protein potentiates STAT1-dependent activation of IFNgamma-responsive promoters in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 91–96. [Google Scholar] [CrossRef]
- Tolstrup, A.B.; Bejder, A.; Fleckner, J.; Justesen, J. Transcriptional regulation of the interferon-gamma-inducible tryptophanyl-tRNA synthetase includes alternative splicing. J. Biol. Chem. 1995, 270, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Marks, M.S.; Driggers, P.H.; Ozato, K. Interferon consensus sequence-binding protein, a member of the interferon regu-latory factor family, suppresses interferon-induced gene transcription. Mol. Cell Biol. 1993, 13, 588–599. [Google Scholar] [PubMed]
- Kadoya, A.; Tone, S.; Maeda, H.; Minatogawa, Y.; Kido, R. Gene structure of human indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 1992, 189, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Koh, I.; Yamazaki, T.; Oomori, Y.; Mukai, Y.; Sugimoto, J. Indoleamine 2,3-dioxygenase and trophoblast invasion in caesare-an scar pregnancy: Implications for the aetiopathogenesis of placenta accreta spectrum. J. Reprod. Immunol. 2020, 138, 103099. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.P.; Studena, K.; Sargent, K.; Redman, C.W. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Beloni, L.; Livi, C.; Maggi, E.; Scarselli, G.; Romagnani, S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998, 4, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Sakai, M.; Sasaki, Y.; Tanebe, K.; Tsuda, H.; Michimata, T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin. Exp. Immunol. 1999, 117, 550–555. [Google Scholar] [CrossRef] [PubMed]

| Normal Pregnancy (n = 12) | Pre-Eclampsia (n = 12) | Nonpregnant (n = 9) | |
|---|---|---|---|
| Tryptophan (µM) | 32.7 ± 4.8 | 42.8 ± 6.9 * | 53.0 ± 9.8 †,‡ |
| Kynurenine (µM) | 1.12 ± 0.17 | 1.02 ± 0.22 | 1.17 ± 0.28 |
| Kynurenine/tryptophan | 0.034 ± 0.004 | 0.024 ± 0.005 * | 0.022 ± 0.004 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, Y.; Sugimoto, J. The Role of the Placental Enzyme Indoleamine 2,3-Dioxygenase in Normal and Abnormal Human Pregnancy. Int. J. Mol. Sci. 2024, 25, 4577. https://doi.org/10.3390/ijms25084577
Kudo Y, Sugimoto J. The Role of the Placental Enzyme Indoleamine 2,3-Dioxygenase in Normal and Abnormal Human Pregnancy. International Journal of Molecular Sciences. 2024; 25(8):4577. https://doi.org/10.3390/ijms25084577
Chicago/Turabian StyleKudo, Yoshiki, and Jun Sugimoto. 2024. "The Role of the Placental Enzyme Indoleamine 2,3-Dioxygenase in Normal and Abnormal Human Pregnancy" International Journal of Molecular Sciences 25, no. 8: 4577. https://doi.org/10.3390/ijms25084577






