The Impact of Normobaric Hypoxia and Intermittent Hypoxic Training on Cardiac Biomarkers in Endurance Athletes: A Pilot Study
Abstract
:1. Introduction
2. Results
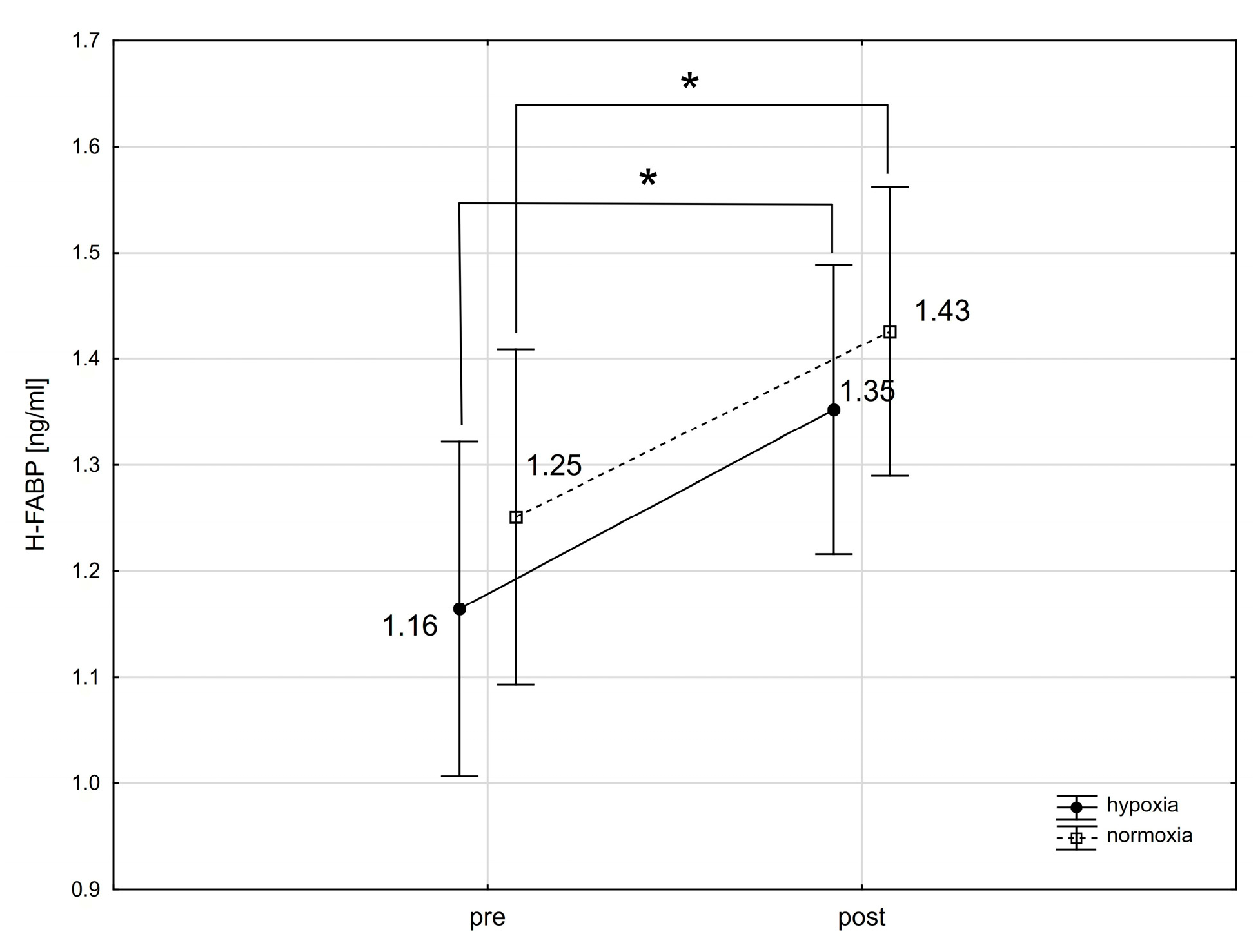
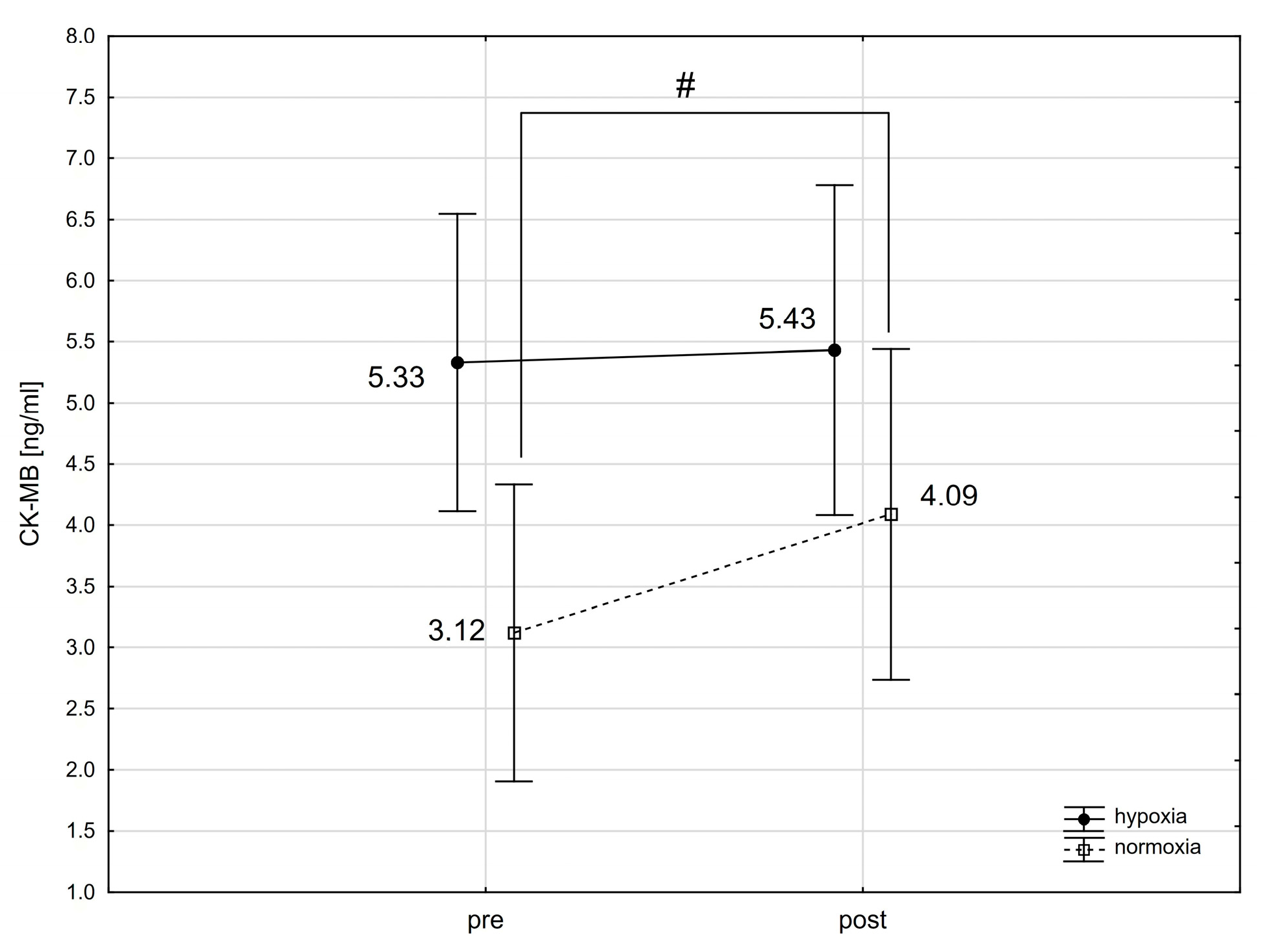
2.1. The Effect of a Single Intense Interval Exercise Session in Normoxia and Hypoxia on the Concentration of Cardiac Markers in the Blood
2.2. The Effect of Training in Normoxia and Hypoxia on the Resting Concentration of Cardiac Markers in the Blood
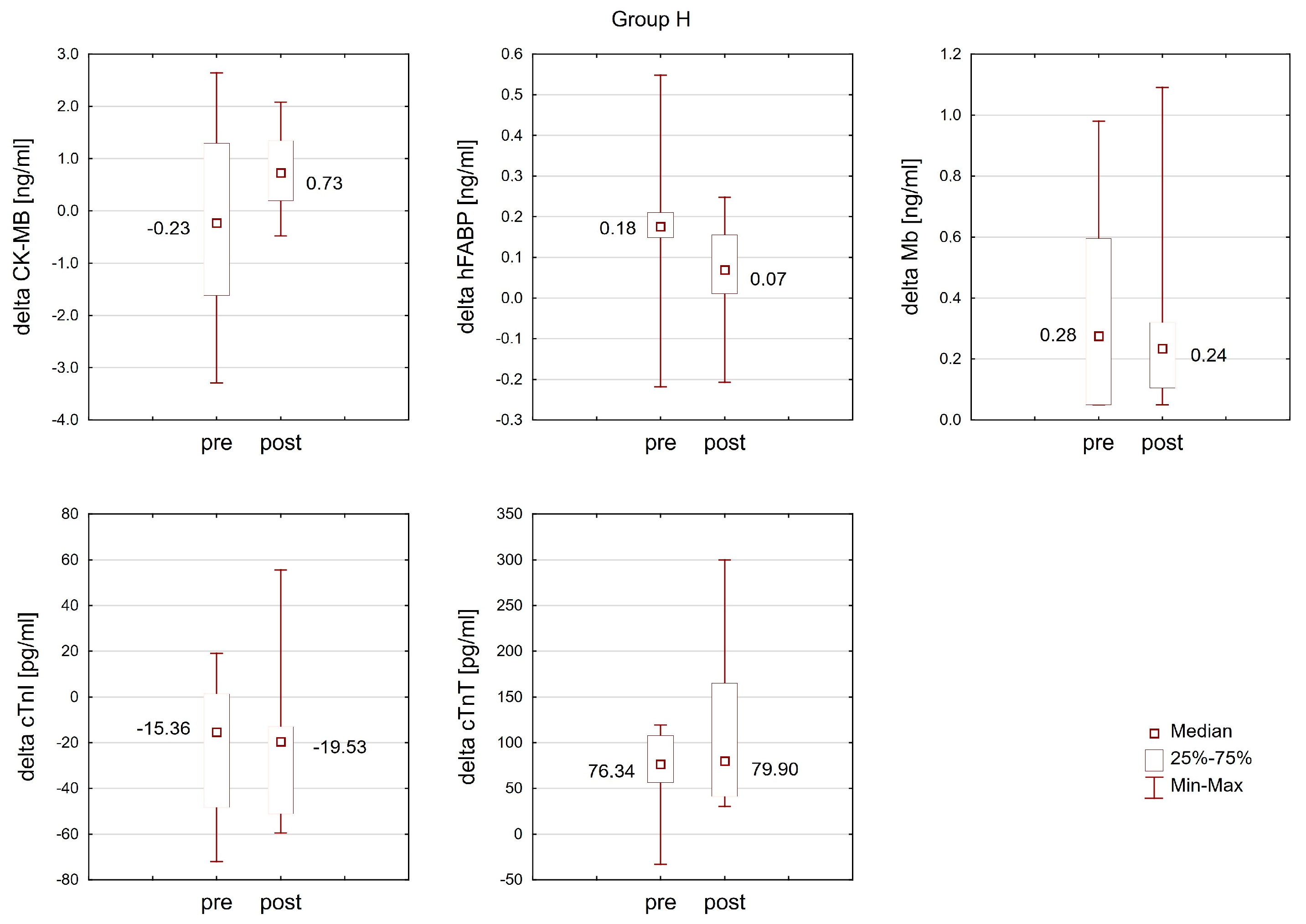
2.3. The Effect of Training in Normoxia and Hypoxia on the Response of Cardiac Markers to Interval Exercise
3. Discussion
3.1. Troponins (cTnT and cTnI)
3.2. Cardiac Isoenzyme of Creatine Kinase (CK-MB)
3.3. Heart-Type Fatty Acid-Binding Protein (H-FABP)
3.4. Mioglobin (Mb)
3.5. Study Limitations
4. Materials and Methods
4.1. Participants
4.2. Study Design
4.3. Measures
4.4. Training Protocol
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Statistical analysis | |
| ES | the effect size |
| F | F-value |
| Me | median |
| p | p-value |
| Q1 | lower quartiles |
| Q3 | upper quartiles |
| SD | standard deviation |
| x | arithmetic mean |
| Cardiac biomarkers | |
| CK-MB | creatine kinase-MB isoenzyme |
| CN/NFAT | calcineurin nuclear factor of activated T-cells |
| cTnI | troponin I |
| cTnT | troponin T |
| H-FABP | heart-type fatty acid-binding protein |
| MB | myoglobin |
| mtCK | creatine kinase isoforms, including mitochondrial |
| NFAT | nuclear factor of activated T-cells |
| Physiological variables | |
| BF | breathing frequency |
| FiO2 | fraction of inspired oxygen |
| HR | heart rate |
| VCO2 | exhaled carbon dioxide |
| VE | minute ventilation |
| VO2 | oxygen uptake |
| VO2max | maximal oxygen uptake |
| VO2maxhyp | maximal oxygen uptake in hypoxia |
| WRmax | maximal workload |
| Ppeak | peak power during Wingate test for upper limbs |
| Pmean | mean power during Wingate test for upper limbs |
| ΔPV% | plasma volume changes |
| Training | |
| EN2 | endurance training (75–85% HR LT) |
| EN3 | endurance training (95–105% HR LT) |
| IHT | intermittent hypoxic training |
| Pmean | mean power during Wingate test for upper limbs |
| Ppeak | peak power during Wingate test for upper limbs |
| REC | recovery training |
| S1 | first test series |
| S2 | second test series |
| SP1 | anaerobic capacity training |
| ST | core stability training |
| TL4 | training in the lab—4 circuits |
| TL5 | training in the lab—5 circuits |
References
- Amann, M.; Eldridge, M.W.; Lovering, A.T.; Stickland, M.K.; Pegelow, D.F.; Dempsey, J.A. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J. Physiol. 2006, 575, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, J.E.; Rusko, H.K.; Rantamaki, J.; Sweins, K.; Nittymaki, S.; Vitasalo, J.T. Effects of oxygen fraction in inspired air on force production and electromyogram activity during ergometer rowing. Eur. J. Appl. Physiol. 1997, 76, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Clark, C.M.; Holden, J.E.; Stanley, C.; Ugurbil, K.; Menon, R.S. 31P magnetic resonance spectroscopy of the Sherpa hart: A phosphocreatine/adenosine triphosphate signature of metabolism defense against hypobaric hypoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Overturf, M.L. Effects of moderate hypertension on cardiac function and metabolism in rabit. Hypertension 1988, 11, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.F.; Sconekess, B.O.; Henning, S.L.; English, D.R.; Lopaschuk, G.D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am. J. Physiol. 1994, 267, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2015, 6, 331–351. [Google Scholar]
- Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog. Cardiovasc. Dis. 2010, 52, 456–466. [Google Scholar] [CrossRef]
- Ezzati, M.; Horwitz, M.E.; Thomas, D.S.; Friedman, A.B.; Roach, R.; Clark, T.; Murray, C.J.; Honigman, B. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: National population-based analysis of US counties. J. Epidemiol. Community Health 2012, 66, e17. [Google Scholar] [CrossRef]
- Burtscher, M. Lower mortality rates in those living at moderate altitude. Aging 2016, 8, 2603–2604. [Google Scholar] [CrossRef]
- Baibas, N.; Trichopoulou, A.; Voridis, E.; Trichopoulos, D. Residance in moutainous compared with lowland areas in relation to total and coronary moralisty. A study in rural Greece. J. Epidemiol. Community Health 2005, 59, 274–278. [Google Scholar] [CrossRef]
- Faeh, D.; Gutzwiller, F.; Bopp, M. Group SNCS. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation 2009, 120, 495. [Google Scholar] [CrossRef] [PubMed]
- Thielke, S.; Slatore, C.G.; Banks, W.A. Association between Alzheimer dementia mortality rate and altitude in California counties. JAMA Psychiatry 2015, 72, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Płoszczyca, K.; Langfort, J.; Czuba, M. The Effects of Altitude Training on Erythropoietic Response and Hematological Variables in Adult Athletes: A Narrative Review. Front. Physiol. 2018, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Bril, G.; Płoszczyca, K.; Piotrowicz, Z.; Chalimoniuk, M.; Roczniok, R.; Zembroń-Łacny, A.; Gerasimuk, D.; Langfort, J. Intermittent Hypoxic Training at Lactate Threshold Intensity Improves Aiming Performance in Well-Trained Biathletes with Little Change of Cardiovascular Variables. BioMed Res. Int. 2019, 2019, 1287506. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Płoszczyca, K.; Kaczmarczyk, K.; Langfort, J.; Gajda, R. Chronic Exposure to Normobaric Hypoxia Increases Testosterone Levels and Testosterone/Cortisol Ratio in Cyclists. Int. J. Environ. Res. Public Health 2022, 19, 5246. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Stray-Gundersen, J. “Living high-training low”: Effect of moderate-altitude acclimatization with low-altitude training on performance. J. Appl. Physiol. 1997, 83, 102–112. [Google Scholar] [CrossRef]
- Townsend, N.E.; Gore, C.J.; Ebert, T.R.; Martin, D.T.; Hahn, A.G.; Chow, C.M. Ventilatory acclimatization is beneficial for high-intensity exercise at altitude in elite cyclists. Eur. J. Sport Sci. 2016, 16, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Fidos-Czuba, O.; Płoszczyca, K.; Zając, A.; Langfort, J. Comparison of the effect of intermittent hypoxic training vs. the live high, train low strategy on aerobic capacity and sports performance in cyclists in normoxia. Biol. Sport 2018, 35, 39–48. [Google Scholar] [PubMed]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic Use of Exercising in Hypoxia: Promises and Limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef]
- Siegel, A.J.; Silverman, L.M.; Holman, B.R. Elevated creatine kinase MB isoenzyme levels in marathon runners. Normal myocardial scintigrams suggest noncardiac source. JAMA 1981, 246, 2049–2051. [Google Scholar] [CrossRef]
- Shave, D.; Davson, E.; Whyte, G.; George, K.; Ball, D.; Collinson, P.; Gaze, D. The cardiospecificity of the third-generation cTnT assay after exercise-induced muscle damage. Med. Sci. Sports Exerc. 2002, 34, 651–654. [Google Scholar] [PubMed]
- König, D.; Schumacher, Y.O.; Heinrich, L.; Schmid, A.; Berg, A.; Dickhuth, H.H. Myocardial stress after competitive exercise in professional road cyclists. Med. Sci. Sports Exerc. 2003, 35, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Tong, T.K.; Shi, Q.; Lin, H.; Zhao, J.; Tian, Y. Serum cardiac troponin response in adolescents playing basketball. Int. J. Sports Med. 2008, 29, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Wedin, J.O.; Henriksson, A.E. Postgame elevation of cardiac markers among elite floorball players. Scand. J. Med. Sci. Sports 2015, 25, 495–500. [Google Scholar] [CrossRef]
- Weippert, M.; Divchev, D.; Schmidt, P.; Gettel, H.; Neugebauer, A.; Behrens, K.; Wolfarth, B.; Braumann, K.M.; Nienaber, C.A. Cardiac troponin T and echocardiographic dimensions after repeated sprint vs. moderate intensity continuous exercise in healthy young males. Sci. Rep. 2016, 6, 24614. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Whyte, G.; Stephenson, C.; Shave, R.; Dawson, E.; Edwards, B.; Gaze, D.; Collinson, P. Postexercise left ventricular function and cTnT in recreational marathon runners. Med. Sci. Sports Exerc. 2004, 36, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Nalcakan, G.R. The Effects of Sprint Interval vs. Continuous Endurance Training on Physiological and Metabolic Adaptations in Young Healthy Adults. J. Hum. Kinet. 2014, 44, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Gaze, D.C. Ischemia modified albumin: A novel biomarker for detection of cardiac ischemia. Drug Metab. Pharmacokinet. 2009, 24, 333–341. [Google Scholar] [CrossRef]
- Marchel, M.; Filipiak, K.J. Contemporary biochemical diagnostics of heart failure in search of new markers. Pol. Prz. Kardiol. 2003, 5, 397–407. [Google Scholar]
- Akbari, A.; Mojtahedi, H.; Rajaei, F.; Marandi, M. The comparison of effects of three types of resistance, endurance and concurrent training on amount of growth hormone secretion in active males. Br. J. Sports Med. 2010, 44, i42. [Google Scholar] [CrossRef]
- Li, F.; Nie, J.; Zhang, H.; Fu, F.; Yi, L.; Hopkins, W.; Liu, Y.; Lu, Y. Effects of Matched Intermittent and Continuous Exercise on Changes of Cardiac Biomarkers in Endurance Runners. Front. Physiol. 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Haram, P.M.; Loennechen, J.P.; Osnes, J.B.; Skomedal, T.; Wisløff, U.; Ellingsen, Ø. Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc. Res. 2005, 67, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, Y.; Nie, J.; Fu, F.H. Effects of acute, intermittent exercise in hypoxic environments on the release of cardiac troponin. Scand. J. Med. Sci. Sports 2016, 26, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, L.J.; Luyten, P.; van der Linden, N.; Urgel, K.; Snijders, D.P.; Knackstedt, C.; Dennert, R.; Kietselaer, B.L.; Mingels, A.M.; Cardinaels, E.P.; et al. Cardiac Troponin T and I Release after a 30-km Run. Am. J. Cardiol. 2016, 118, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Skadberg, Ø.; Kleiven, Ø.; Ørn, S.; Bjørkavoll-Bergseth, M.F.; Melberg, T.H.; Omland, T.; Aakre, K.M. The cardiac troponin response following physical exercise in relation to biomarker criteria for acute myocardial infarction; the North Sea Race Endurance Exercise Study (NEEDED) 2013. Clin. Chim. Acta 2018, 479, 155–159. [Google Scholar] [CrossRef]
- Mehta, R.; Gaze, D.; Mohan, S.; Williams, K.L.; Sprung, V.; George, K.; Jeffries, R.; Hudson, Z.; Perry, M.; Shave, R. Postexercise cardiac troponin release is related to exercise training history. Int. J. Sports Med. 2012, 33, 333–337. [Google Scholar] [PubMed]
- Nie, J.; George, K.P.; Tong, T.K.; Gaze, D.; Tian, Y.; Lin, H.; Shi, Q. The influence of a half-marathon race upon cardiac troponin T release in adolescent runners. Curr. Med. Chem. 2011, 18, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.; Boos, C.; Holdsworth, D.; Begley, J.; Hall, D.; Lumley, A.; Burnett, A.; Hawkins, A.; O’Hara, J.; Ball, S.; et al. Cardiac biomarkers at high altitude. High Alt. Med. Biol. 2014, 15, 452–458. [Google Scholar] [CrossRef]
- Middleton, N.; George, K.; Whyte, G.; Gaze, D.; Collinson, P.; Shave, R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J. Am. Coll. Cardiol. 2008, 52, 1813–1814. [Google Scholar] [CrossRef]
- Tian, Y.; Nie, J.; Huang, C.; George, K.P. The kinetics of highly sensitive cardiac troponin T release after prolonged treadmill exercise in adolescent and adult athletes. J. Appl. Physiol. 2012, 113, 418–425. [Google Scholar] [CrossRef]
- Legaz-Arrese, A.; López-Laval, I.; George, K.; Puente-Lanzarote, J.J.; Mayolas-Pi, C.; Serrano-Ostáriz, E.; RevillaMartí, P.; Moliner-Urdiales, D.; Reverter-Masià, J. Impact of an endurance training program on exercise-induced cardiac biomarker release. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H913–H920. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Lee, Y.H.; Chae, J.H.; Kim, C.K. Creatine kinase isoenzyme activity during and after an ultra-distance (200 km) run. Biol. Sport 2015, 32, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, H.; Monazami, A.A.; Azizi, M. Effects of Acute Resistance Training on Biochemical Markers of Myocardial Injury (cTnT, cTnI, CK-MB) in Non-Athlete Women. J. Kermanshah Univ. Med. Sci. 2019, 23, e84103. [Google Scholar] [CrossRef]
- Hazar, M.; Otag, A.; Otag, I.; Sezen, M.; Sever, O. Effect of increasing maximal aerobic exercise on serum muscles enzymes in professional field hockey players. Glob. J. Health Sci. 2014, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ertel, K.A.; Hallam, J.E.; Hillman, A.R. The effects of training status and exercise intensity on exercise-induced muscle damage. J. Sports Med. Phys. Fit. 2020, 60, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bekkelund, S.I. Leisure physical exercise and creatine kinase activity. The Tromsø study. Scand. J. Med. Sci. Sports 2020, 30, 2437–2444. [Google Scholar] [CrossRef]
- Waskova-Arnostova, P.; Kasparova, D.; Elsnicova, B.; Novotny, J.; Neckar, J.; Kolar, F.; Zurmanova, J. Chronic Hypoxia Enhances Expression and Activity of Mitochondrial Creatine Kinase and Hexokinase in the Rat Ventricular Myocardium. Cell. Physiol. Biochem. 2014, 33, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.; Nicolay, K.; Wieringa, B.; Saks, V.; Wallimann, T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J. Biol. Chem. 2000, 275, 6937–6944. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.; Whittington, H.J.; Ostrowski, P.J.; Cao, F.; Tyler, J.; Lake, H.A.; Neubauer, S.; Lygate, C.A. Increasing creatine kinase activity protects against hypoxia/reoxygenation injury but not against anthracycline toxicity in vitro. PLoS ONE 2017, 12, e0182994. [Google Scholar] [CrossRef]
- Saito, T.; Matsumoto, H.; Matsuyama, H.; Sakai, Y.; Yamashita, K.; Kishi, K.; Morishita, Y. Clinical evaluation of the new creatine kinase MB reagent kit “L-System CK-MB MtO”. Rinsho Byori 2011, 59, 236–242. [Google Scholar]
- Fournier, N.C.; Richard, M.A. Role of fatty acid-binding protein in cardiac fatty acid oxidation. Mol. Cell. Biochem. 1990, 98, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, A.J.; Nellis, S.; Neely, J.R. Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ. Res. 1978, 43, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Apstein, C.S.; Saouf, R.; Brecher, P. Leakage of heart fatty acid binding protein with ischemia and reperfusion in the rat. J. Mol. Cell. Cardiol. 1989, 21, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Prasad, M.R.; Das, D.K. Modulation of fatty acid-binding capacity of heart fatty acid-binding protein by oxygen-derived free radicals. Mol. Cell. Biochem. 1990, 98, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Tanaka, T.; Somiya, K.; Tsuji, R.; Okamoto, F.; Kawamura, K.; Ohkaru, Y.; Asayama, K.; Ishii, H. Human heart-type cytoplasmic fatty acid-binding protein as an indicator of acute myocardial infarction. Heart Vessels 1995, 10, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Alhadi, H.A.; Fox, K.A. Do we need additional markers of myocyte necrosis: The potential value of hear fatty-acid-binding protein. QJM 2004, 97, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Sorichter, S.; Mair, J.; Koller, A.; Pelsers, M.M.; Puschendorf, B.; Glatz, J.F. Early assessment of exercise induced skeletal muscle injury using plasma fatty acid binding protein. Br. J. Sports Med. 1998, 32, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C. Influence of acute physical exercise on emerging muscular biomarkers. Clin. Chem. Lab. Med. 2008, 46, 1313–1318. [Google Scholar] [CrossRef]
- Ari, H.; Tokaç, M.; Alihanoğlu, Y.; Kıyıcı, A.; Kayrak, M.; Arı, M.; Sönmez, O.; Gök, H. Relationship between heart-type fatty acidbinding protein levels and coronary artery disease in exercise stress testing: An observational study. Anadolu Kardiyol. Derg. 2011, 1, 685–691. [Google Scholar]
- Sbarouni, E.; Georgiadou, P.; Koutelou, M.; Constantinos, M.; Chaidaroglou, A.; Degiannis, D.; Voudris, V. Heart type fatty acid binding protein in relation to pharmacologic scintigraphy in coronary artery disease. Clin. Chem. Lab. Med. 2011, 50, 387–390. [Google Scholar] [CrossRef]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE 2013, 8, e81318. [Google Scholar] [CrossRef] [PubMed]
- Saheed, U.N.; Meludu, S.C.; Onuora, I.J.; Obi-Ezeani, C.N.; Dioka, C.E.; Njoku, C.M. Endurance physical activity impact on heart type fatty acid binding protein of health individuals in eastern Nigeria. Glob. J. Med. Public Health 2018, 7, 2277–9604. [Google Scholar]
- Sponder, M.; Lichtenauer, M.; Wernly, B.; Paar, V.; Hoppe, U.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Strametz-Juranek, J. Serum heart-type fatty acid-binding protein decreases and soluble isoform of suppression of tumorigenicity 2 increases significantly by long-term physical activity. J. Investig. Med. 2019, 67, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Munjal, D.D.; McFadden, J.A.; Matix, P.A.; Coffman, K.D.; Cattaneo, S.M. Changes in serum myoglobin, total creatine kinase, lactate dehydrogenase and creatine kinase MB levels in runners. Clin. Biochem. 1983, 16, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Roxin, L.E.; Hedin, G.; Venge, P. Muscle cell leakage of myoglobin after long-term exercise and relation to the individual performances. Int. J. Sports Med. 1986, 7, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Cipryan, L. IL-6, Antioxidant Capacity and Muscle Damage Markers Following High-Intensity Interval Training Protocols. J. Hum. Kinet. 2017, 56, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Spada, T.C.; Silva, J.M.R.D.; Francisco, L.S.; Marçal, L.J.; Antonangelo, L.; Zanetta, D.M.T.; Yu, L.; Burdmann, E.A. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS ONE 2018, 13, e0205791. [Google Scholar] [CrossRef] [PubMed]
- Scalco, R.S.; Snoeck, M.; Quinlivan, R.; Treves, S.; Laforét, P.; Jungbluth, H.; Voermans, N.C. Exertional rhabdomyolysis: Physiological response or manifestation of an underlying myopathy? BMJ Open Sport Exerc. Med. 2016, 7, e000151. [Google Scholar] [CrossRef] [PubMed]
- Furman, J. When exercise causes exertional rhabdomyolysis. JAAPA 2015, 28, 38–43. [Google Scholar] [CrossRef]
- Reynafarje, B. Myoglobin Content and Enzymatic Activity of Human Skeletal Muscle—Their Relation with the Process of Adaptation to High Altitude; Technical Documentary Reporti No. 62-89; Armed Services Technical Information Agency: Arlington, VA, USA, 1962; 8p. [Google Scholar]
- Kanatous, S.B.; Mammen, P.P.; Rosenberg, P.B.; Martin, C.M.; White, M.D.; Dimaio, J.M.; Huang, G.; Muallem, S.; Garry, D.J. Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am. J. Physiol. Cell. Physiol. 2009, 296, C393–C402. [Google Scholar] [CrossRef]
- Shave, R.; Dawson, E.; Whyte, G.; George, K.; Gaze, D.; Collinson, P. Effect of prolonged exercise in a hypoxic environment on cardiac function and cardiac troponin T. Br. J. Sports Med. 2004, 38, 86–88. [Google Scholar] [CrossRef]
- Jacobs, I.; Esbjörnsson, M.; Sylvén, C.; Holm, I.; Jansson, E. Sprint training effects on muscle myoglobin, enzymes, fiber types, and blood lactate. Med. Sci. Sports Exerc. 1987, 19, 368–374. [Google Scholar] [CrossRef]
- Terrados, N.; Jansson, E.; Sylvén, C.; Kaijser, L. Is hypoxia a stimulus for synthesis of oxidative enzymes and myoglobin? J. Appl. Physiol. 1990, 68, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Okazaki, K.; Kuno, S.; Asano, K.; Shimojo, H.; Katsuta, S. Endurance training under 2500-m hypoxia does not increase myoglobin content in human skeletal muscle. Eur. J. Appl. Physiol. 2001, 85, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, B.A. Both hypoxia and work are required to enhance expression of myoglobin in skeletal muscle. Focus on “Hypoxia reprograms calcium signaling and regulates myoglobin expression”. Am. J. Physiol. Cell. Physiol. 2009, 296, C390–C392. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.K.; Lambert, J.P.; Chow, C.W.; Lefer, D.J.; Calvert, J.W. Chronic exercise downregulates myocardial myoglobin and attenuates nitrite reductase capacity during ischemia-reperfusion. J. Mol. Cell. Cardiol. 2013, 64, 1–10. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]




| Group H | Group N | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| cTnT (pg/mL) | 44.4 ± 33.6 | 60.8 ± 31.5 | 44.8 ± 39.7 | 29.3 ± 18.5 |
| cTnI (pg/mL) | 125.9 ± 85.9 | 159.4 ± 69.6 | 43.7 ± 12.6 | 114.3 ± 67.7 |
| Mb (ng/mL) | 0.26 ± 0.14 | 0.23 ± 0.18 | 0.36 ± 0.18 | 0.18 ± 0.09 * |
| H-FABP (ng/mL) | 1.17 ± 0.35 | 1.06 ± 0.23 | 1.26 ± 0.24 | 0.18 ± 0.09 * |
| CK-MB (ng/mL) | 4.75 ± 1.70 | 2.13 ± 0.77 *** | 3.15 ± 2.21 | 2.52 ± 1.68 |
| Variable | Group H (n = 8) | Group N (n = 8) |
|---|---|---|
| Age (years) | 19.1 ± 1.3 | 20.5 ± 1.3 |
| Body height (m) | 1.83 ± 0.03 | 1.81 ± 0.04 |
| Body mass (kg) | 76.4 ± 5.4 | 74.1 ± 6.3 |
| Body fat (%) | 9.3 ± 3.5 | 9.5 ± 1.6 |
| VO2max (mL/kg/min) | 56.0 ± 4.0 | 53.7 ± 5.8 |
| WRmax (W) | 362.0 ± 22.2 | 352.7 ± 33.1 |
| Ppeak (W) | 741.0 ± 124.3 | 784.6 ± 149.5 |
| Pmean (W) | 452.7 ± 37.6 | 435.1 ± 35.4 |
| Day | Microcyle 1 | Microcyle 2 | Microcyle 3 | Microcyle 4 |
|---|---|---|---|---|
| 1 |
|
|
|
|
| 2 |
|
|
|
|
| 3 |
|
|
|
|
| 4 |
|
|
|
|
| 5 |
|
|
|
|
| 6 |
|
|
|
|
| 7 | off | off | off | off |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goliniewski, J.; Czuba, M.; Płoszczyca, K.; Chalimoniuk, M.; Gajda, R.; Niemaszyk, A.; Kaczmarczyk, K.; Langfort, J. The Impact of Normobaric Hypoxia and Intermittent Hypoxic Training on Cardiac Biomarkers in Endurance Athletes: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 4584. https://doi.org/10.3390/ijms25094584
Goliniewski J, Czuba M, Płoszczyca K, Chalimoniuk M, Gajda R, Niemaszyk A, Kaczmarczyk K, Langfort J. The Impact of Normobaric Hypoxia and Intermittent Hypoxic Training on Cardiac Biomarkers in Endurance Athletes: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(9):4584. https://doi.org/10.3390/ijms25094584
Chicago/Turabian StyleGoliniewski, Jakub, Miłosz Czuba, Kamila Płoszczyca, Małgorzata Chalimoniuk, Robert Gajda, Adam Niemaszyk, Katarzyna Kaczmarczyk, and Józef Langfort. 2024. "The Impact of Normobaric Hypoxia and Intermittent Hypoxic Training on Cardiac Biomarkers in Endurance Athletes: A Pilot Study" International Journal of Molecular Sciences 25, no. 9: 4584. https://doi.org/10.3390/ijms25094584







