Elucidating the Role of circTIAM1 in Guangling Large-Tailed Sheep Adipocyte Proliferation and Differentiation via the miR-485-3p/PLCB1 Pathway
Abstract
:1. Introduction
2. Results
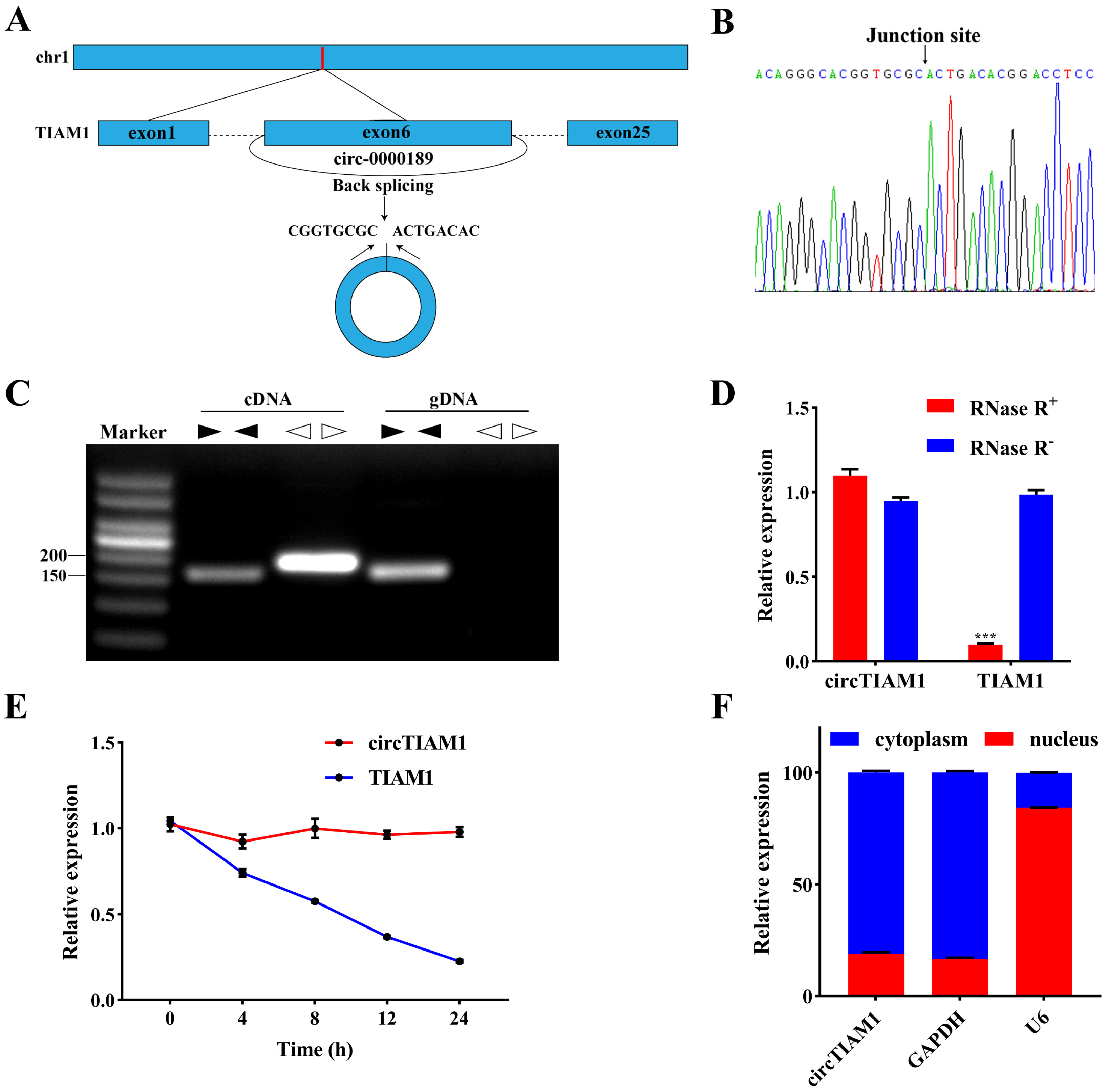
2.1. Characterization of circTIAM1 in Ovine Adipocytes
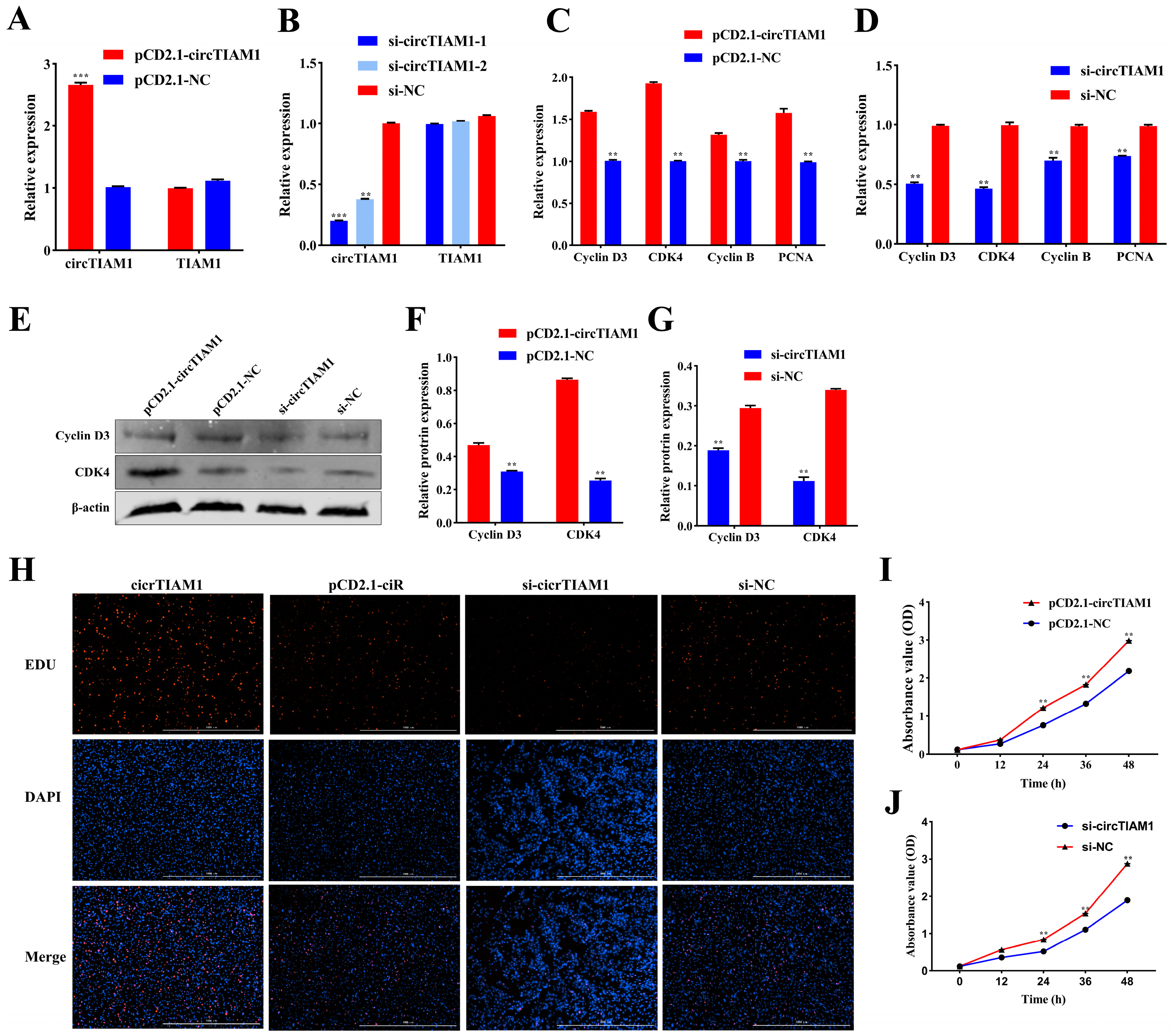
2.2. circTIAM1 Promotes Adipocyte Proliferation
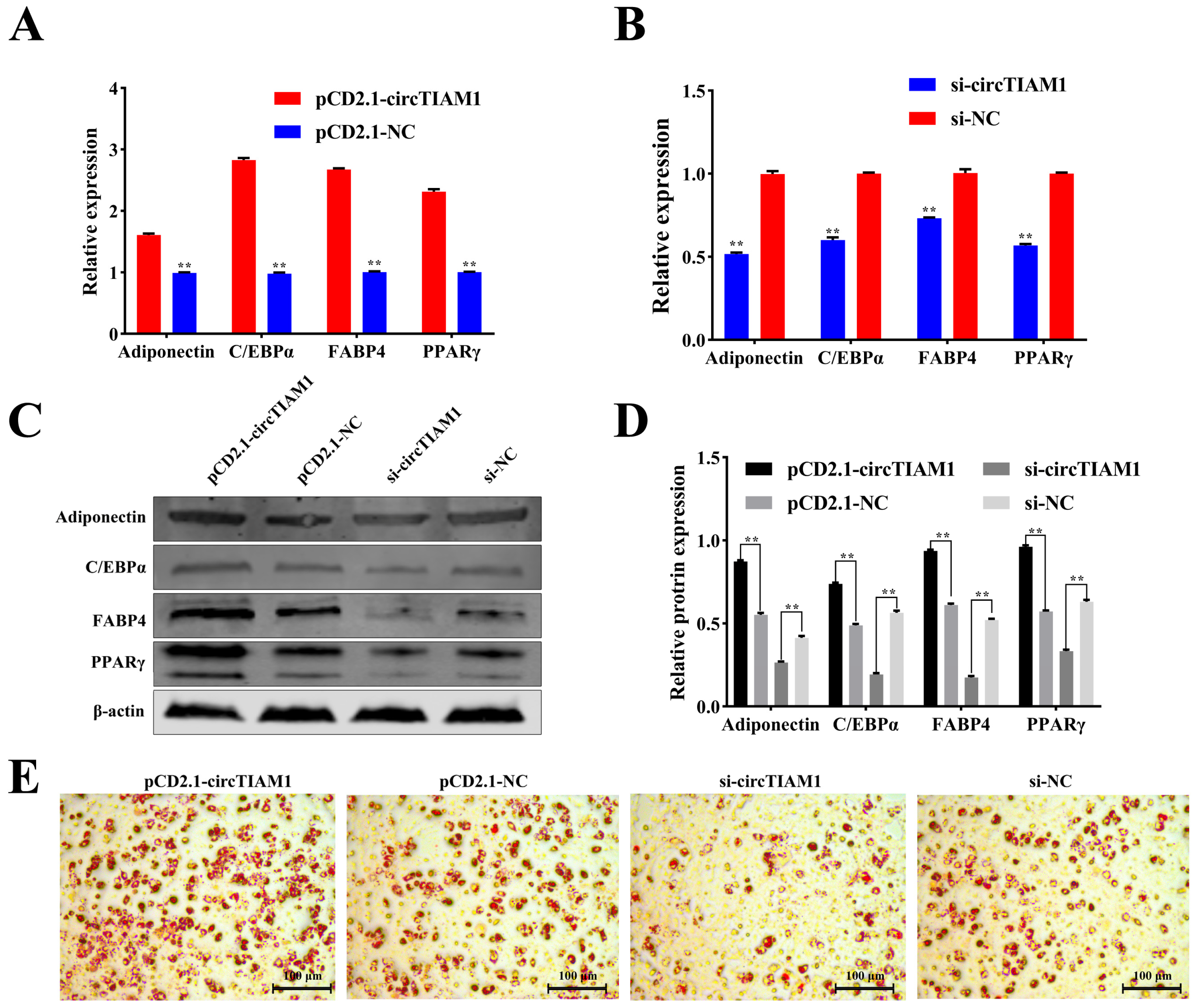
2.3. circTIAM1 Promotes Adipocytes Differentiation
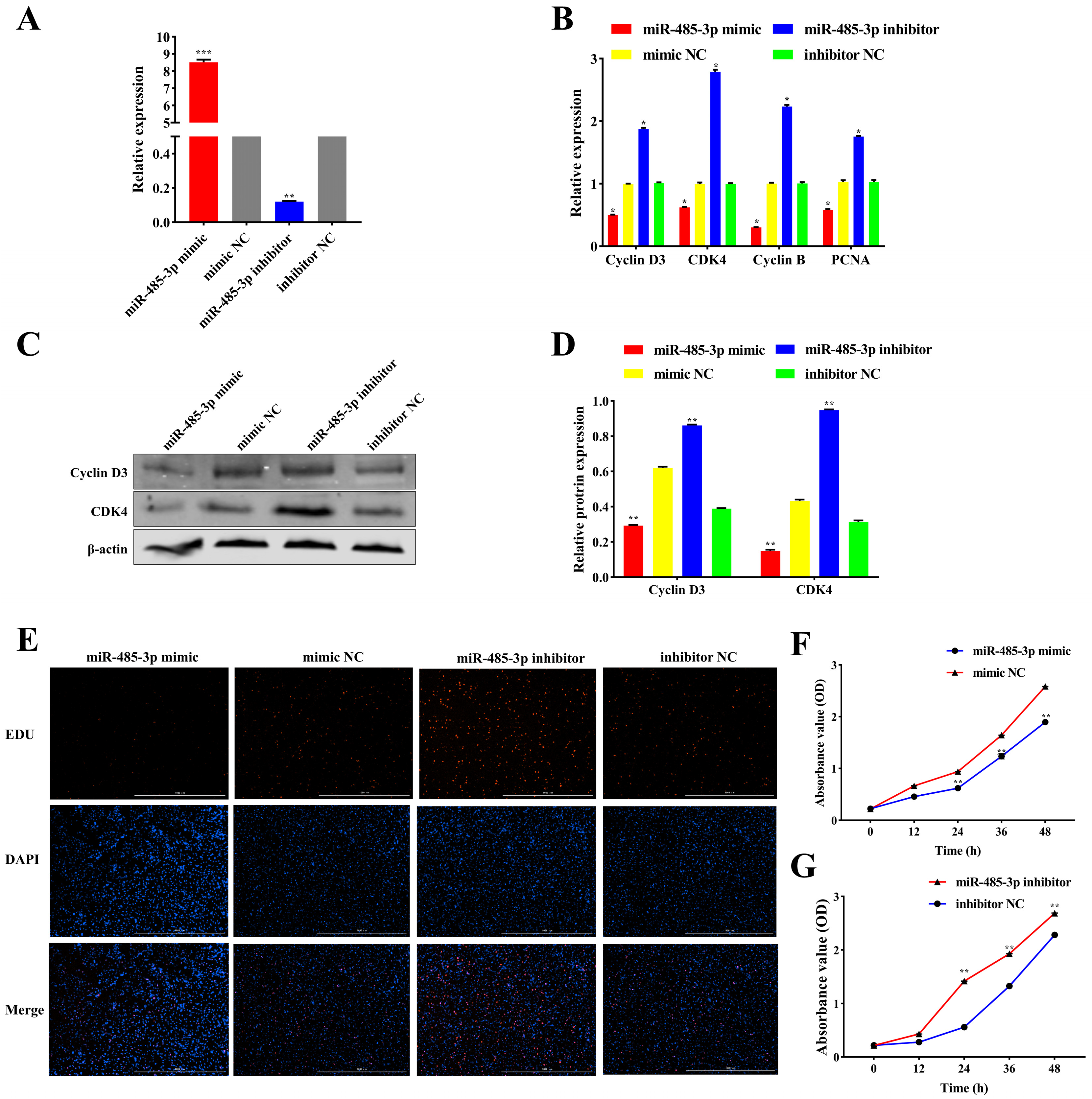
2.4. miR-485-3p Inhibits Adipocyte Proliferation
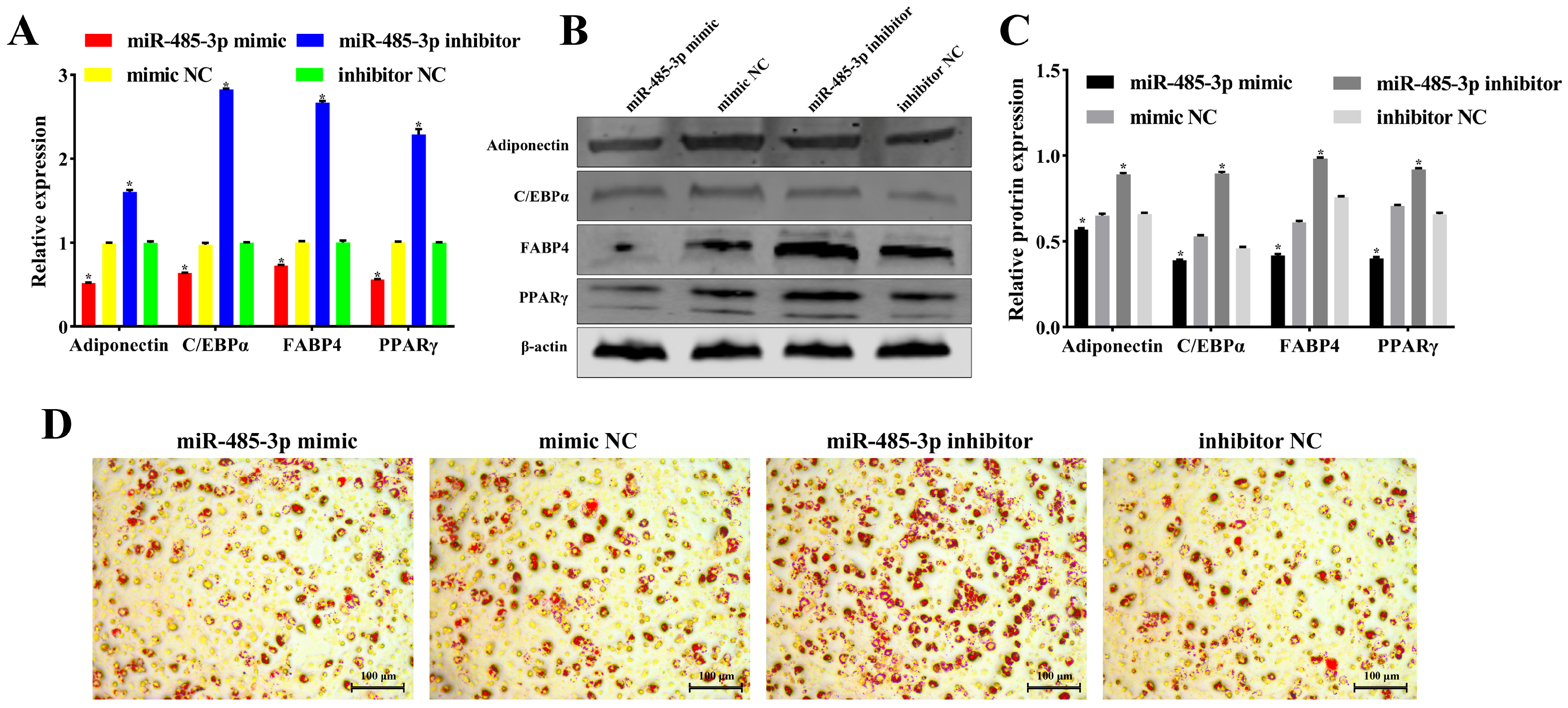
2.5. miR-485-3p Inhibits Adipocyte Differentiation
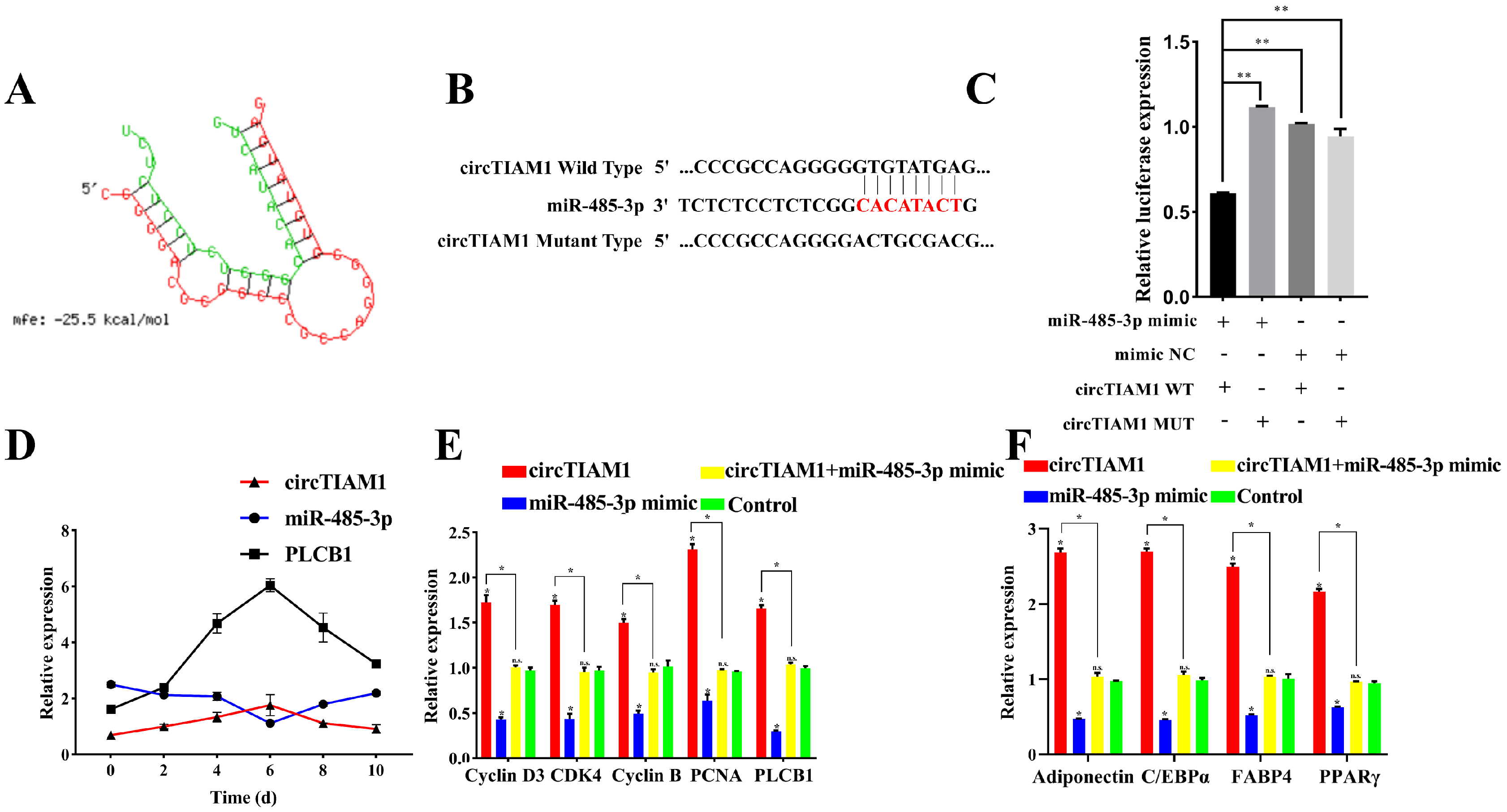
2.6. PLCB1: A Target of miR-485-3p
2.7. circTIAM1 Serves as a Molecular Sponge for miR-485-3p
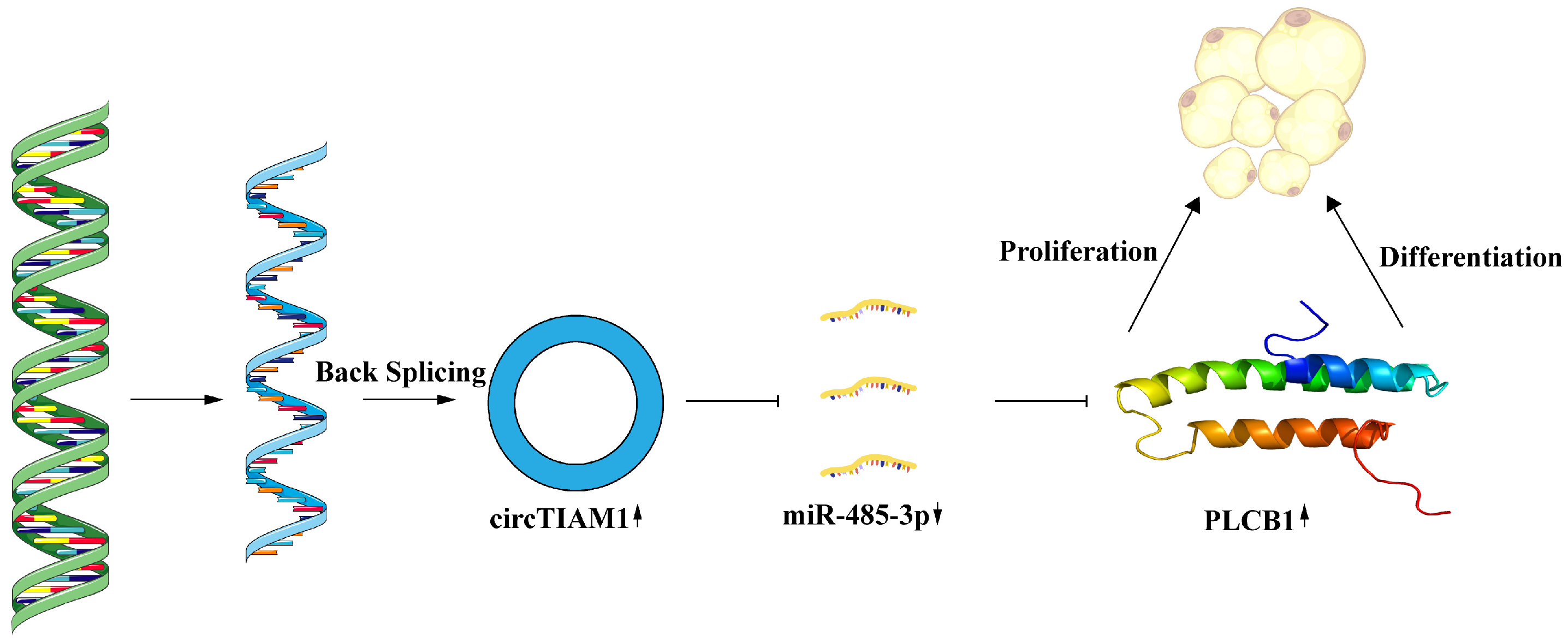
3. Discussion
4. Materials and Methods
4.1. Ethical Approval Declarations and Sample Collection
4.2. Cell Isolation and Culture
4.3. Vector Construction and Cell Transfection
4.4. Cell Transfection
4.5. RNase R Treatment
4.6. Actinomycin D Assay
4.7. RNA and Genomic DNA Extraction and Quantitative Real-Time PCR
4.8. EdU and CCK-8 Analysis
4.9. Western Blotting
4.10. Oil Red Staining
4.11. Binding Relationship Prediction
4.12. Dual-Luciferase Reporter Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.X.; Yang, J.; Lv, F.H.; Hu, X.J.; Xie, X.L.; Zhang, M.; Li, W.R.; Liu, M.J.; Wang, Y.T.; Li, J.Q.; et al. Genomic reconstruction of the history of native sheep reveals the peopling patterns of nomads and the expansion of early pastoralism in east asia. Mol. Biol. Evol. 2017, 34, 2380–2395. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.M.; Ardeshir, N.J.; Mohammad, M.S.; Ken, G.D.; John, C.M. Genomic scan of selective sweeps in thin and fat tail sheep breeds for identifying of candidate regions associated with fat deposition. BMC Genet. 2012, 13, 10. [Google Scholar]
- Marina, N.S.; Quan, N.; Sean, M.; Laercio, R.P.; Ross, T.; Tony, V.; Antonio, R.; Miguel, P.E.; Rudiger, B.; Shannon, C.; et al. Sheep genome functional annotation reveals proximal regulatory elements contributed to the evolution of modern breeds. Nat. Commun. 2018, 9, 859. [Google Scholar]
- Yang, J.; Li, W.R.; Lv, F.H.; He, S.G.; Tian, S.L.; Peng, W.F.; Sun, Y.W.; Zhao, Y.X.; Tu, X.L.; Zhang, M.; et al. Whole-genome sequencing of native sheep provides insights into rapid adaptations to extreme environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Ren, X.; Yang, G.L.; Xie, X.L.; Zhao, Y.X.; Zhang, M.; Shen, Z.Q.; Ren, Y.L.; Gao, L.; Shen, M.; et al. Genome-wide association analysis identifies the genetic basis of fat deposition in the tails of sheep (Ovis aries). Anim. Genet. 2017, 48, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Kohram, H.; Zare Shahneh, A.; Nik-khah, A.; Campbell, A.W. Comparison of the meat quality and fatty acid composition of traditional fat-tailed (Chall) and tailed (Zel) Iranian sheep breeds. Meat Sci. 2012, 92, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat deposition and fat effects on meat quality-A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013, 51, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.M.; Hugouvieux, V.; Nayak, A.; Conos, S.A.; Capovilla, G.; Cildir, G.; Jourdain, A.; Tergaonkar, V.; Schmid, M.; Zubieta, C.; et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants. 2017, 3, 17053. [Google Scholar] [CrossRef] [PubMed]
- Guarnerio, J.; Zhang, Y.; Cheloni, G.; Panella, R.; Mae Katon, J.; Simpson, M.; Matsumoto, A.; Papa, A.; Loretelli, C.; Petri, A.; et al. Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res. 2019, 29, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Rong, D.; Hui, B.; He, X.; Jiang, W.; Xu, Y.; Cao, H.; Xu, Z.; Tang, W. CircETFA upregulates CCL5 by sponging miR-612 and recruiting EIF4A3 to promote hepatocellular carcinoma. Cell Death Discov. 2021, 7, 321. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Y.; Mao, R.; Yang, H.; Zhang, Y.; Zhang, Y.; Guo, P.; Zhan, D.; Zhang, T. Circular RNA SAMD4A controls adipogenesis in obesity through the miR-138-5p/EZH2 axis. Theranostics 2020, 10, 4705–4719. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, E.; Kang, Z.; Bi, Y.; Liu, H.; Xu, H.; Wang, Z.; Lei, C.; Chen, H.; Lan, X. CircRNA profiling reveals an abundant circBDP1 that regulates bovine fat development by sponging miR-181b/miR-204 targeting Sirt1/TRARG1. J. Agric. Food Chem. 2022, 70, 14312–14328. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, Y.; Cui, R.; He, S.; Zhao, X.; Wu, K.; Fang, M. Sus_circPAPPA2 regulates fat deposition in castrated pigs through the miR-2366/GK pathway. Biomolecules 2022, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.C.-Y.; Wang, Y.; Lu, A.Z.; Gomez-Salazar, M.A.; Xu, J.; Li, D.; Meyers, C.; Negri, S.; Wangsiricharoen, S.; Broderick, K.; et al. TIAM1 acts as an actin organization regulator to control adipose tissue–derived pericyte cell fate. JCI Insight 2023, 8, e159141. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Shao, R.; Li, M.; Yan, Q.; Hu, H. MiR-485-3p modulates neural stem cell differentiation and proliferation via regulating TRIP6 expression. J. Cell. Mol. Med. 2020, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Mongiorgi, S.; Ramazzotti, G.; Follo, M.Y.; Mariani, G.A.; Suh, P.G.; McCubrey, J.A.; Cocco, L.; Manzoli, L. Nuclear inositide signaling via phospholipase C. J. Cell. Biochem. 2017, 118, 1969–1978. [Google Scholar] [CrossRef]
- Mount, P.; Davies, M.; Choy, S.W.; Cook, N.; Power, D. Obesity-related chronic kidney disease-the role of lipid metabolism. Metabolites 2015, 5, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Guo, Y.; Yuan, F.; Chen, S.; Yin, H.; Jiang, X.; Jiao, F.; Wang, F.; Ji, H.; Hu, G.; et al. Autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening. Autophagy 2020, 16, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Integrated regulation of the cellular metabolism and function of immune cells in adipose tissue. Clin. Exp. Pharmacol. Physiol. 2016, 43, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in circular RNA and its applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.; Deng, Y.; Li, Z.; Tang, A. CircRNA-mediated regulation of brown adipose tissue adipogenesis. Front. Nutr. 2022, 9, 926024. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yousuf, S.; Liu, T.; Zhang, X.; Huang, W.; Li, A.; Xie, L.; Miao, X. The comprehensive detection of miRNA and circRNA in the regulation of intramuscular and subcutaneous adipose tissue of Laiwu pig. Sci. Rep. 2022, 12, 16542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Gan, M.L.; Shen, L.Y.; Niu, L.L.; Guo, Z.Y.; Wang, J.Y.; Zhang, S.H.; Zhu, L. circRNA on animal skeletal muscle development regulation. Yi Chuan 2020, 42, 1178–1191. [Google Scholar] [PubMed]
- Lv, X.; Chen, W.; Sun, W.; Hussain, Z.; Chen, L.; Wang, S.; Wang, J. Expression profile analysis to identify circular RNA expression signatures in hair follicle of Hu sheep lambskin. Genomics 2020, 112, 4454–4462. [Google Scholar] [CrossRef] [PubMed]
- Errichelli, L.; Dini Modigliani, S.; Laneve, P.; Colantoni, A.; Legnini, I.; Capauto, D.; Rosa, A.; De Santis, R.; Scarfò, R.; Peruzzi, G.; et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017, 8, 14741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tao, L.; Xu, N.; Lu, X.; Wang, J.; He, G.; Tang, Q.; Huang, K.; Shen, S.; Chu, J. CircRNA circTIAM1 promotes papillary thyroid cancer progression through the miR-646/HNRNPA1 signaling pathway. Cell Death Discov. 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Z.; Ye, H.; Sun, X.; Zhang, H.; Sun, Y.; Mao, Y.; Yang, Z.; Li, M. Emerging functions of circular RNA in the regulation of adipocyte metabolism and obesity. Cell Death Discov. 2022, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.; Ershov, N.; Damarov, I.; Merkulova, T. SNPs in 3′UTR miRNA target sequences associated with individual drug susceptibility. Int. J. Mol. Sci. 2022, 23, 13725. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Guan, L.; Qi, Q.; Shu, G.; Jiang, Q.; Yuan, L.; Xi, Q.; Zhang, Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-α (TNF-α) in the porcine model. PLoS ONE 2013, 8, e71568. [Google Scholar] [CrossRef] [PubMed]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory mechanism of microRNA expression in cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, Y.; Qiao, L.; Xia, D.; Pan, Y.; Liu, W. MiR-128-1-5p regulates differentiation of ovine stromal vascular fraction by targeting the KLF11 5′-UTR. Domest. Anim. Endocrinol. 2022, 80, 106711. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, L.J.; Liu, H.; Song, Y.J.; Yang, Q.Q.; Liu, Y.; Qian, S.W.; Tang, Q.Q. Exosomal miR-27b-3p secreted by visceral adipocytes contributes to endothelial inflammation and atherogenesis. Cell Rep. 2023, 42, 111948. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Billi, A.M.; Mongiorgi, S.; Ratti, S.; McCubrey, J.A.; Suh, P.G.; Cocco, L.; Ramazzotti, G. Nuclear phosphatidylinositol signaling: Focus on phosphatidylinositol phosphate kinases and phospholipases C. J. Cell. Physiol. 2016, 231, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, M.; Blalock, W.L.; Bavelloni, A.; Faenza, I.; Raffini, M.; Tagliavini, F.; Manzoli, L.; Cocco, L. PI-PLCβ1b affects Akt activation, cyclin E expression, and caspase cleavage, promoting cell survival in pro-B-lymphoblastic cells exposed to oxidative stress. FASEB J. 2015, 29, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Lukinovic-Skudar, V.; Donlagic, L.; Banfíc, H.; Visnjic, D. Nuclear phospholipase C-beta1b activation during G2/M and late G1 phase in nocodazole-synchronized HL-60 cells. Biochim. Biophys. Acta 2005, 1733, 148–156. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Mitchell, M.D.; Faenza, I.; Cocco, L.; Gilmour, R.S. Nuclear PLCbeta1 is required for 3T3-L1 adipocyte differentiation and regulates expression of the cyclin D3-cdk4 complex. Cell Signal. 2009, 21, 926–935. [Google Scholar] [CrossRef]
- Sarruf, D.A.; Iankova, I.; Abella, A.; Assou, S.; Miard, S.; Fajas, L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2005, 25, 9985–9995. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, H.; Yang, J.; Hao, D.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Lin, F.; et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017, 8, e3153. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Feng, X.; Ma, Y.; Wei, D.; Zhang, L.; Wang, S.; Ma, Y. CircADAMTS16 inhibits differentiation and promotes proliferation of bovine adipocytes by targeting miR-10167-3p. Cells 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]

| Name | Sequences (5′-3′) |
|---|---|
| si-circTIAM1-1 | AGGGCACGGTGCGCACTGA |
| si-circTIAM1-2 | TGCGCACTGACACGGACCT |
| Names | Sequences (5′-3′) |
|---|---|
| sh-PLCB1 forward | GATCCGCACCCAAGGACCCTAAATTATTCAAGAGATAATTTAGGGTCCTTGGGTGCTTTTTTG |
| sh-PLCB1 reverse | AATTCAAAAAAGCACCCAAGGACCCTAAATTATCTCTTGAATAATTTAGGGTCCTTGGGTGCG |
| sh-PLCB1-2 forward | GATCCGCGTGGATTCATCTAACTATATTCAAGAGATATAGTTAGATGAATCCACGCTTTTTTG |
| sh-PLCB1-2 reverse | AATTCAAAAAAGCGTGGATTCATCTAACTATATCTCTTGAATATAGTTAGATGAATCCACGCG |
| Name of Primers | GenBank Accession Number | Sequences (5′-3′) |
|---|---|---|
| circTIAM1 forward | GCCGTCAAGAACTTCCTGGTGC | |
| circTIAM1 reverse | GACAGGAGGTCCGTGTCAGTGG | |
| PPARγ forward | NM_001100921.1 | ATCTTGACGGGAAAGACGAC |
| PPARγ reverse | AAACTGACACCCCTGGAAGAT | |
| FABP4 forward | NM_001114667.1 | AAACTGGGATGGGAAATCAACC |
| FABP4 reverse | TGCTCTCTCGTAAACTCTGGTAGC | |
| Adiponectin forward | NM_001308565.1 | ATCCCCGGGCTGTACTACTT |
| Adiponectin reverse | CTGGTCCACGTTCTGGTTCT | |
| C/EBPα forward | NM_001308574.1 | TCCGTGGACAAGAACAGCAA |
| C/EBPα reverse | TCATTGTCACTGGTCAGCTCC | |
| β-Actin forward | NM_001009784.3 | TGATGATATTGCTGCGCTCG |
| β-Actin reverse | GGGTCAGGATGCCTCTCTTG | |
| miR-485-3p | GUCAUACACGGCUCUCCUCUCU | |
| U6 forward | XR_003587591.1 | CTCGCTTCGGCAGCACA |
| U6 reverse | AACGCTTCACGAATTTGCGT | |
| PLCB1 forward | XM_042230393.1 | TGGAGCTGGAGCAAGAATACC |
| PLCB1 reverse | AGAGCTGTGATTGCTGTCTTCA | |
| Cyclin B forward | XM_060400336.1 | CGTACTCCGTCTCCAGCC |
| Cyclin B reverse | AGCCAGTCAATCAGGATGGC | |
| Cyclin D3 forward | XM_027958330.3 | ACAGGCAGCATCGGAGCTGC |
| Cyclin D3 reverse | CTTTGGGCGCTGGGCTGGGA | |
| PCNA forward | XM_004014340.5 | ATCAGCTCAAGTGGCGTGAA |
| PCNA reverse | TGCCAAGGTGTCCGCATTAT | |
| CDK4 forward | XM_012158548.4 | CCAATGTTGTCCGGCTGATG |
| CDK4 reverse | CCTTGATCGTTTCGGCTGG |
| Name | Sequences (5′-3′) |
|---|---|
| circTIAM1 convergent | F: GGTAGCGAGTTTGCCGACAG |
| R: AATTCTCATACACCCCCTGGC | |
| circTIAM1 divergent | F: GCCGTCAAGAACTTCCTGGTGC |
| R: GACAGGAGGTCCGTGTCAGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zhao, B.; Shen, Y.; Peng, M.; Qiao, L.; Liu, J.; Pan, Y.; Yang, K.; Liu, W. Elucidating the Role of circTIAM1 in Guangling Large-Tailed Sheep Adipocyte Proliferation and Differentiation via the miR-485-3p/PLCB1 Pathway. Int. J. Mol. Sci. 2024, 25, 4588. https://doi.org/10.3390/ijms25094588
Liang Y, Zhao B, Shen Y, Peng M, Qiao L, Liu J, Pan Y, Yang K, Liu W. Elucidating the Role of circTIAM1 in Guangling Large-Tailed Sheep Adipocyte Proliferation and Differentiation via the miR-485-3p/PLCB1 Pathway. International Journal of Molecular Sciences. 2024; 25(9):4588. https://doi.org/10.3390/ijms25094588
Chicago/Turabian StyleLiang, Yu, Bishi Zhao, Yan Shen, Miao Peng, Liying Qiao, Jianhua Liu, Yangyang Pan, Kaijie Yang, and Wenzhong Liu. 2024. "Elucidating the Role of circTIAM1 in Guangling Large-Tailed Sheep Adipocyte Proliferation and Differentiation via the miR-485-3p/PLCB1 Pathway" International Journal of Molecular Sciences 25, no. 9: 4588. https://doi.org/10.3390/ijms25094588






