Verification of AKT and CDK5 Gene and RNA Interference Combined with Irradiation to Mediate Fertility Changes in Plutella xylostella (Linnaeus)
Abstract
:1. Introduction
2. Results
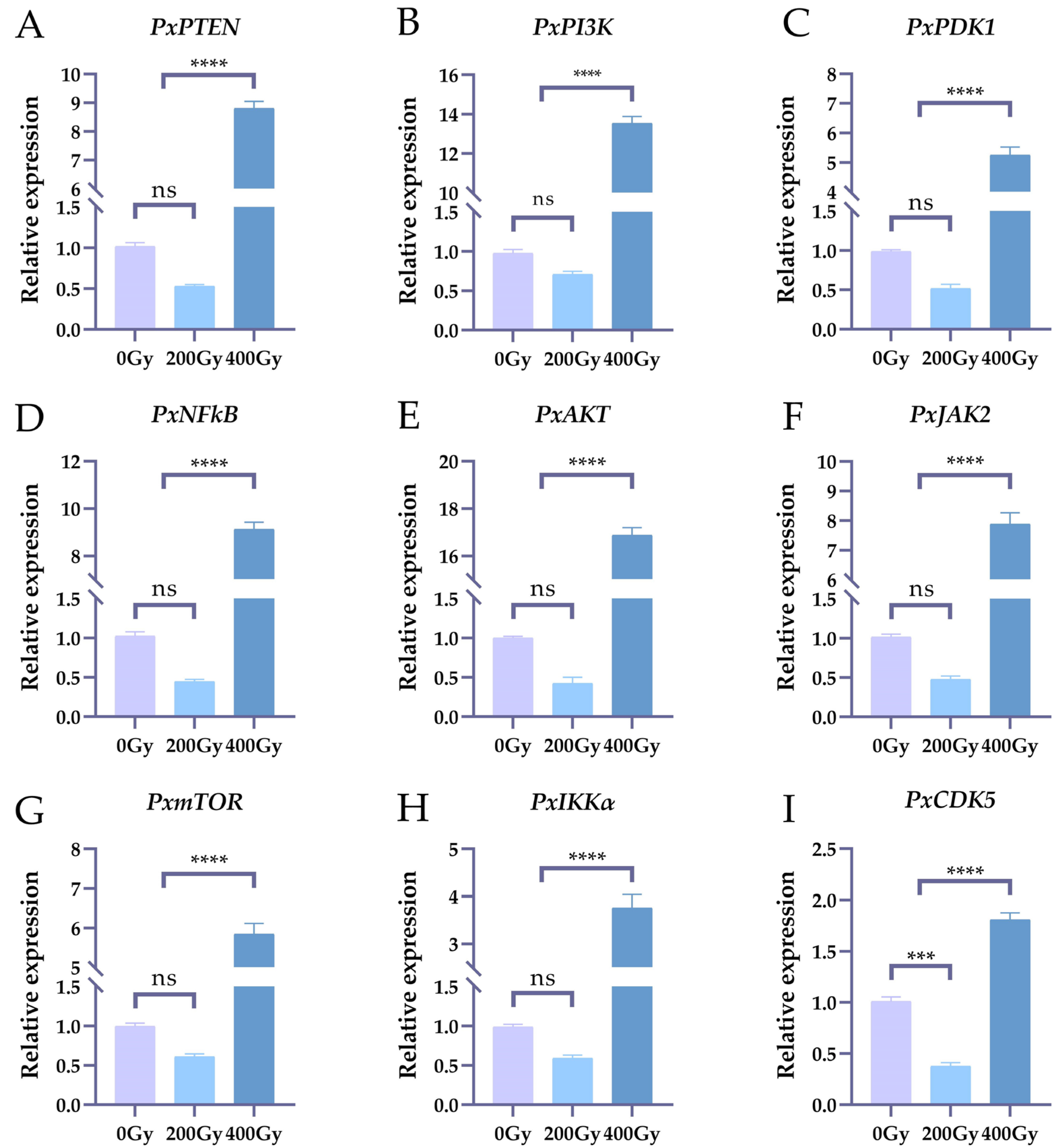
2.1. The Effects on the Expression Levels of Nine Genes of Irradiation
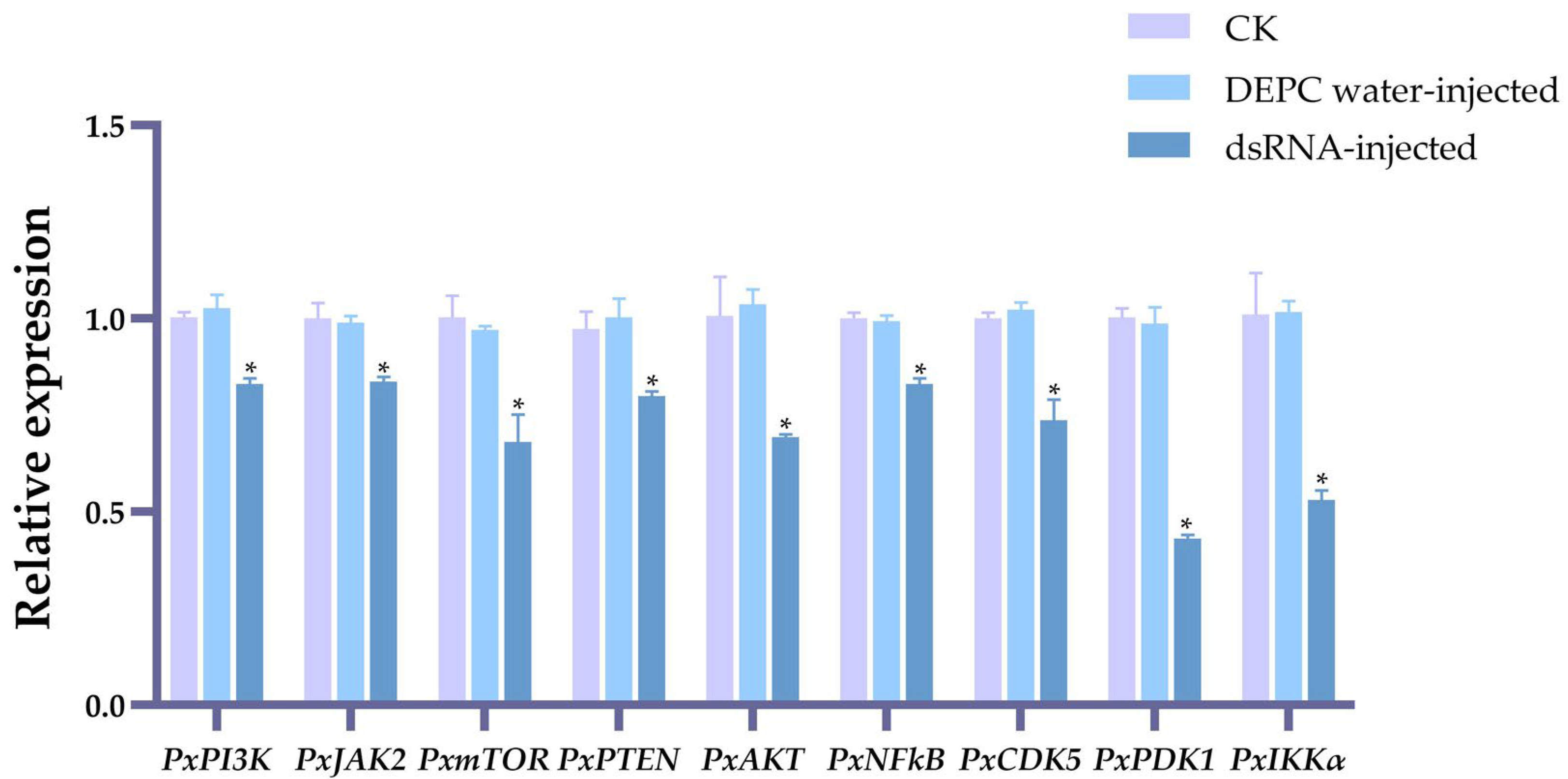
2.2. The Effects on Plutella xylostella Reproductive Indicators of Gene-Knockdown
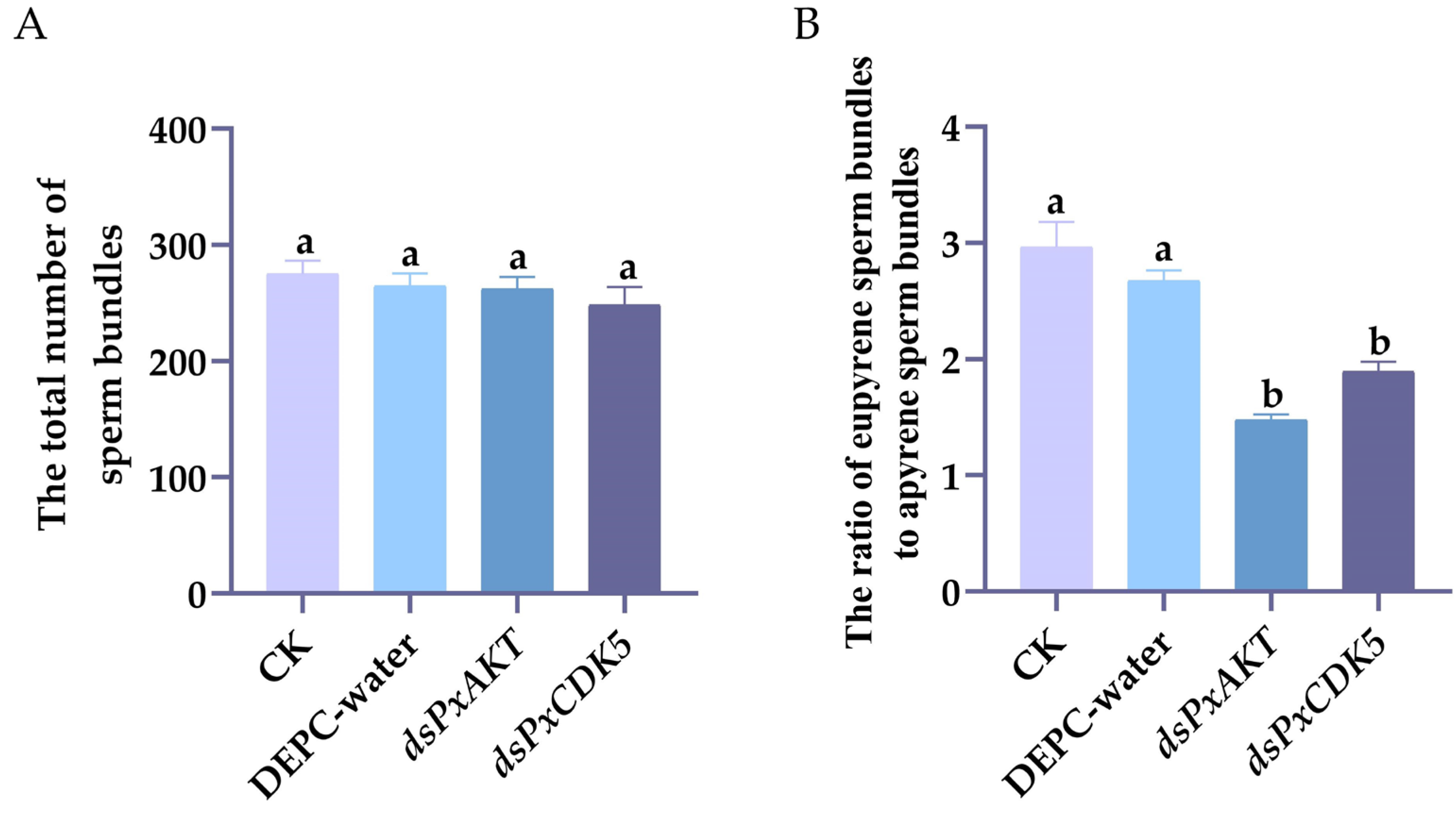
2.3. The Effects of PxAKT and PxCDK5 Knockdown on Plutella xylostella Sperm Bundles
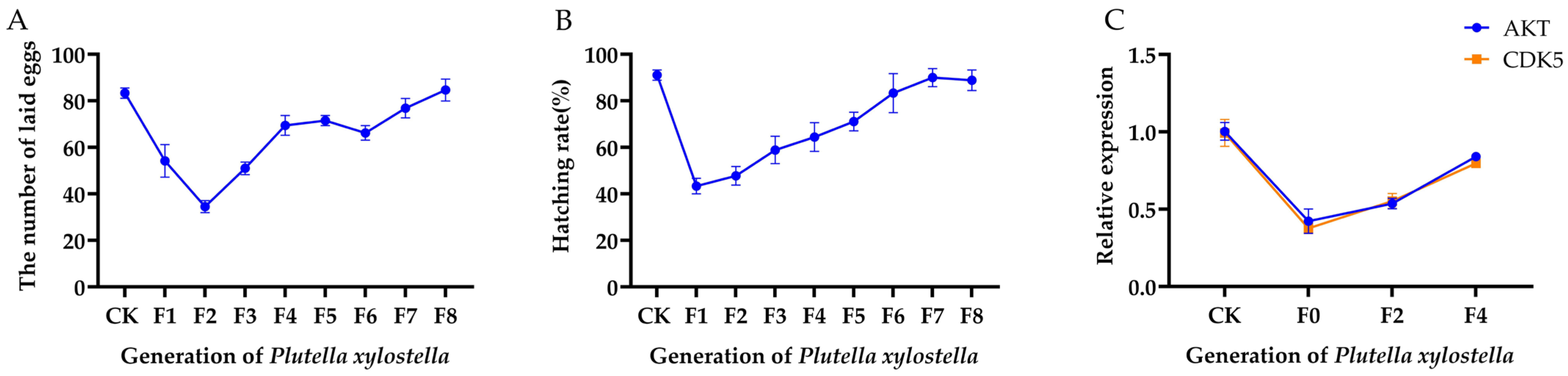
2.4. The Alterations in PxAKT and PxCDK5 Expression Levels and in the Fertility of Plutella xylostella across Generations Following Irradiation Treatment
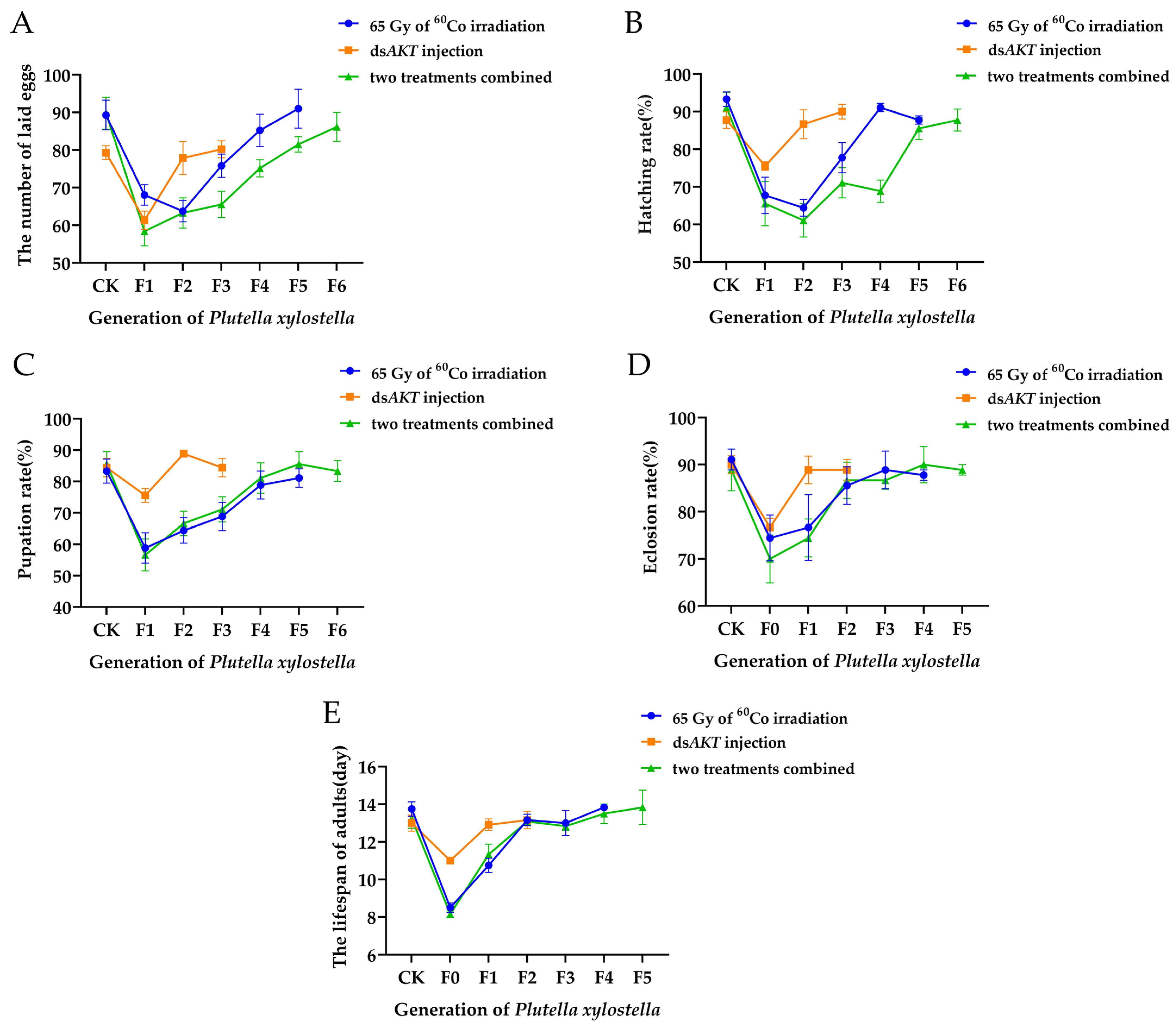
2.5. The Impact of PxAKT Injection Combined with Low-Dose Irradiation on Plutella xylostella Fertility
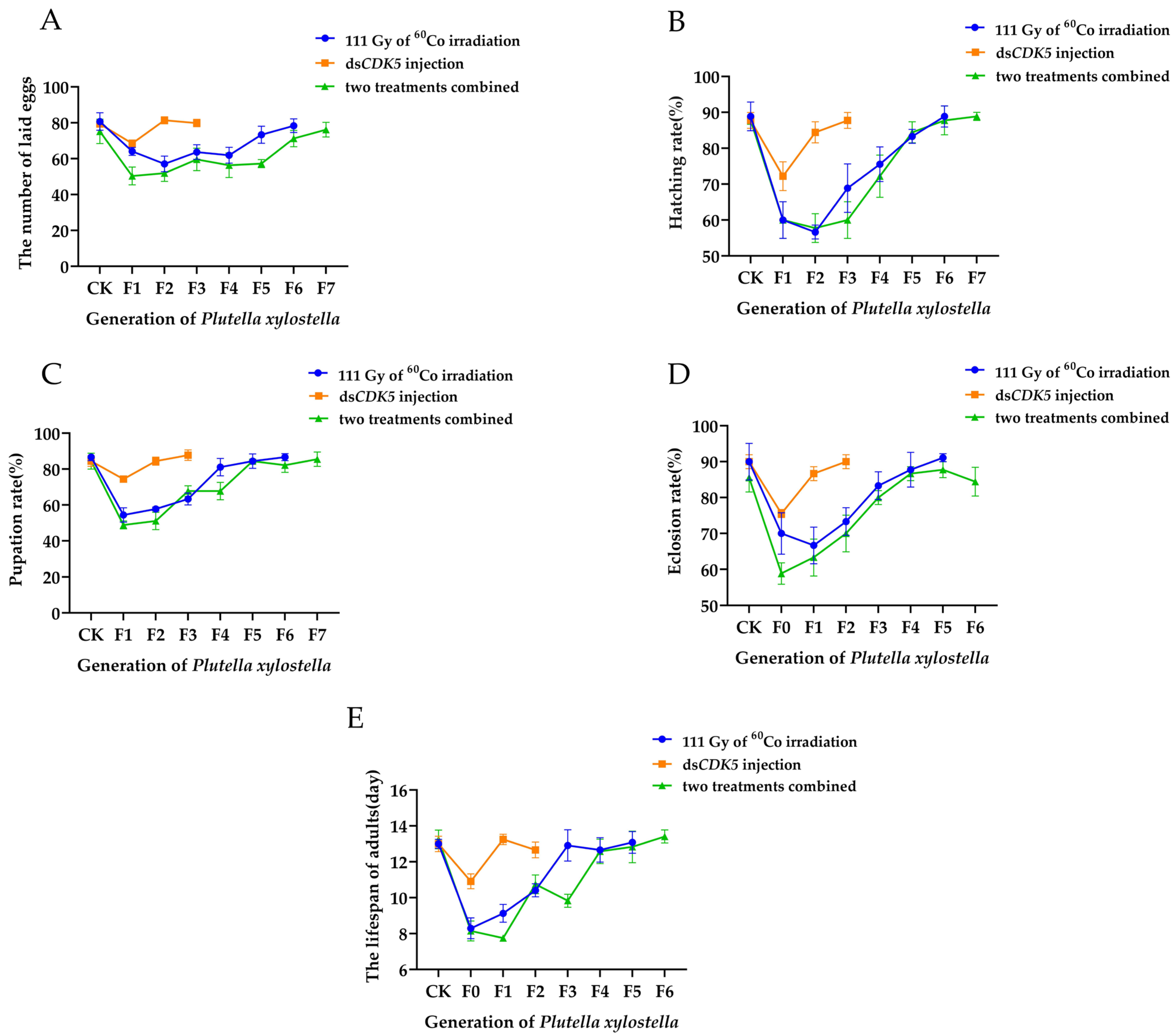
2.6. The Effects of Low-Dose Irradiation Combined with PxCDK5 Knockdown on the Fertility of Plutella xylostella
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. 60Co-γ Irradiation of Male Pupae
4.3. Bioassay of the Effects on Fecundity and Vitality
4.3.1. Oviposition and Egg Hatchability
4.3.2. Pupation Rate, Emergence Rate and Adult Lifespan
4.4. Dissection and Observation of Testes
4.5. Measurements of the Number of Sperm Bundles and the Weight of the Testes
4.6. Total RNA Extraction and cDNA Synthesis
4.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. RNA Interference, Including dsRNA Synthesis and dsRNA Injecting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Feng, X.; Liu, S.-S.; You, M.; Furlong, M.J. Biology, Ecology, and Management of the Diamondback Moth in China. Annu. Rev. Entomol. 2016, 61, 277–296. [Google Scholar] [CrossRef]
- Dancau, T.; Mason, P.G.; Cappuccino, N. Elusively Overwintering: A Review of Diamondback Moth (Lepidoptera: Plutellidae) Cold Tolerance and Overwintering Strategy. Can. Entomol. 2018, 150, 156–173. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chen, Y.-T.; Shi, M.-Z.; Li, J.-W.; Xu, R.-B.; Pozsgai, G.; You, M.-S. Spatio-Temporal Distribution Patterns of Plutella xylostella (Lepidoptera: Plutellidae) in a Fine-Scale Agricultural Landscape Based on Geostatistical Analysis. Sci. Rep. 2021, 11, 13622. [Google Scholar] [CrossRef]
- Philips, C.R.; Fu, Z.; Kuhar, T.P.; Shelton, A.M.; Cordero, R.J. Natural History, Ecology, and Management of Diamondback Moth (Lepidoptera: Plutellidae), with Emphasis on the United States. J. Integr. Pest. Manag. 2014, 5, D1–D11. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Mao, K.; You, H.; Li, J. Susceptibility of Field Populations of the Diamondback Moth, Plutella xylostella, to a Selection of Insecticides in Central China. Pestic. Biochem. Physiol. 2016, 132, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.; Xu, H. Monitoring Resistance of Field Populations of Diamondback Moth Plutella xylostella L. (Lepidoptera: Yponomeutidae) to Five Insecticides in South China: A Ten-Year Case Study. Crop Prot. 2011, 30, 272–278. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.; Wang, K.; Song, J.; Zhu, B.; Gao, X.; Liang, P. A Novel V263I Mutation in the Glutamate-Gated Chloride Channel of Plutella xylostella (L.) Confers a High Level of Resistance to Abamectin. Int. J. Biol. Macromol. 2023, 230, 123389. [Google Scholar] [CrossRef]
- Sayani, Z.; Mikani, A.; Mosallanejad, H. Biochemical Resistance Mechanisms to Fenvalerate in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2019, 112, 1372–1377. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Yuan, J.; Wang, S.; Xu, B.; Wang, S.; Zhang, Y.; Wu, Q. Insecticide Resistance Monitoring of the Diamondback Moth (Lepidoptera: Plutellidae) Populations in China. J. Econ. Entomol. 2021, 114, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xue, Y.; Xiao, G.; Xie, M.; Huang, S.; You, S.; Wyckhuys, K.A.G.; You, M. Inheritance and Fitness Costs of Resistance to Bacillus Thuringiensis Toxin Cry2Ad in Laboratory Strains of the Diamondback Moth, Plutella xylostella (L.). Sci. Rep. 2019, 9, 6113. [Google Scholar] [CrossRef]
- Immune Responses to Bacillus Thuringiensis in the Midgut of the Diamondback Moth, Plutella xylostella. Dev. Comp. Immunol. 2020, 107, 103661. [CrossRef] [PubMed]
- Yin, F.; Lin, Q.; Wang, X.; Li, Z.; Feng, X.; Shabbir, M.Z. The Glutathione S-Transferase (PxGST2L) May Contribute to the Detoxification Metabolism of Chlorantraniliprole in Plutella xylostella (L.). Ecotoxicology 2021, 30, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Mendonça, C.M.; de Arruda Mendonça, J.; Dias, E.S.F.; Florêncio, S.G.L.; Guedes, D.R.D.; Paiva, M.H.S.; Amaral, A.; Netto, A.M.; Melo-Santos, M.A.V. Effects of Gamma Radiation on the Reproductive Viability of Aedes aegypti and Its Descendants (Diptera: Culicidae). Acta Trop. 2022, 228, 106284. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, B.A.; Nawaj, A.; Maiga, H.; Soukia, O.; Pagabeleguem, S.; Ouédraogo/Sanon, M.S.G.; Vreysen, M.J.B.; Mach, R.L.; de Beer, C.J. X-rays Are as Effective as Gamma-Rays for the Sterilization of Glossina Palpalis Gambiensis Vanderplank, 1911 (Diptera: Glossinidae) for Use in the Sterile Insect Technique. Sci. Rep. 2023, 13, 17633. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Dias, V.S.; Parker, A.G.; Maiga, H.; Kraupa, C.; Vreysen, M.J.B.; Mamai, W.; Schetelig, M.F.; Somda, N.S.B.; Bouyer, J. Radiation Dose-Rate Is a Neglected Critical Parameter in Dose–Response of Insects. Sci. Rep. 2022, 12, 6242. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and Its Applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Kandul, N.P.; Liu, J.; Sanchez, C.H.M.; Wu, S.L.; Marshall, J.M.; Akbari, O.S. Transforming Insect Population Control with Precision Guided Sterile Males with Demonstration in Flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Staples, D.; Díaz-Fleischer, F.; Montoya, P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Neotrop. Entomol. 2021, 50, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Marec, F.; Vreysen, M.J.B. Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Hofmeyr, J.H.; Hofmeyr, M.; Carpenter, J.E.; Bloem, S.; Slabbert, J.P. Sterile Insect Releases for Control of Thaumatotibia leucotreta (Lepidoptera: Tortricidae): An Assessment on Semi-Commercial Scale. Afr. Entomol. 2016, 24, 80–89. [Google Scholar] [CrossRef]
- Suckling, D.M.; Charles, J.; Allan, D.; Chaggan, A.; Barrington, A.; Burnip, G.M.; El-Sayed, A.M. Performance of Irradiated Teia anartoides (Lepidoptera: Lymantriidae) in Urban Auckland, New Zealand. J. Econ. Entomol. 2005, 98, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a Sterile Insect Release Program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective Overflooding Ratios and Release-Recapture Field Studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef]
- Bloem, S.; Carpenter, J.; Mccluskey, A.; Fugger, R.; Arthur, S.; Wood, S. Suppression of the Codling Moth Cydia Pomonella in British Columbia, Canada Using an Area-Wide Integrated Approach with an SIT Components. In Proceedings of the Area-Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 591–601. [Google Scholar]
- Zhao, J.; Li, S.; Xu, L.; Li, C.; Li, Q.; Dewer, Y.; Wu, K. Effects of X-ray Irradiation on Biological Parameters and Induced Sterility of Ephestia Elutella: Establishing the Optimum Irradiation Dose and Stage. Front. Physiol. 2022, 13, 895882. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, A.; Albayrak, S.; Karaborklu, S. Gamma radiation sensitivity of the eggs, larvae and pupae of Indian meal moth Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Pest. Manag. Sci. 2008, 64, 505–512. [Google Scholar] [CrossRef] [PubMed]
- LingYan, L.; XiaoFeng, Z.; XiangQin, W.; Ke, Z.; QunFang, W. Effect of 60Co-γ radiation on inherited sterility of Plutella xylostella (Lepidoptera: Plutellidae). J. Northwest A F Univ. Nat. Sci. Ed. 2018, 46, 149–154. [Google Scholar]
- Kolliopoulou, A.; Swevers, L. Recent Progress in RNAi Research in Lepidoptera: Intracellular Machinery, Antiviral Immune Response and Prospects for Insect Pest Control. Curr. Opin. Insect Sci. 2014, 6, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA Interference in Lepidoptera: An Overview of Successful and Unsuccessful Studies and Implications for Experimental Design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a Cotton Bollworm P450 Monooxygenase Gene by Plant-Mediated RNAi Impairs Larval Tolerance of Gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Cai, L.-J.; Li, T.-P.; Lin, X.-J.; Huang, Y.-P.; Qin, J.-M.; Xu, W.; You, M.-S. Identification and Characterization of Zero Population Growth (Zpg) Gene in Plutella xylostella. Physiol. Entomol. 2022, 47, 46–54. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Li, Y.; You, M.; Zhao, Q. Functional Analysis of the Orphan Genes Tssor-3 and Tssor-4 in Male Plutella xylostella. J. Integr. Agric. 2021, 20, 1880–1888. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and Sterile Insect Techniques Combined Eliminate Mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, K.; Wen, J.; Zeng, Y.; Deng, Y.; Hu, Q.; Weng, Q. Molecular Mechanism of Male Sterility Induced by 60Co γ-Rays on Plutella xylostella (Linnaeus). Molecules 2023, 28, 5727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Wen, J.; He, M.; Hu, Q.; Zhang, K.; Weng, Q. Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Sterility Induced by Irradiation of Plutella xylostella (Linnaeus). Ecotoxicol. Environ. Saf. 2024, 270, 115890. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, L.; Yang, Y.-F.; Zou, M.-M.; He, W.-Y.; Wang, Y.; Wang, Q.; Vasseur, L.; You, M.-S. Transcriptome Profiling of the Plutella xylostella (Lepidoptera: Plutellidae) Ovary Reveals Genes Involved in Oogenesis. Gene 2017, 637, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Riehle, M.A.; Brown, M.R. Molecular Analysis of the Serine/Threonine Kinase Akt and Its Expression in the Mosquito Aedes aegypti. Insect Mol. Biol. 2003, 12, 225–232. [Google Scholar] [CrossRef] [PubMed]
- De Felici, M.; Klinger, F.G. PI3K/PTEN/AKT Signaling Pathways in Germ Cell Development and Their Involvement in Germ Cell Tumors and Ovarian Dysfunctions. Int. J. Mol. Sci. 2021, 22, 9838. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Díaz, E.S.; Kong, M. Proteasome Activity and Its Relationship with Protein Phosphorylation during Capacitation and Acrosome Reaction in Human Spermatozoa. Soc. Reprod. Fertil. Suppl. 2007, 65, 269–273. [Google Scholar] [PubMed]
- Lee, W.-J.; Jung, E.-J.; Hwang, J.-M.; Bae, J.-W.; Kwon, W.-S. GRP78 Plays a Key Role in Sperm Function via the PI3K/PDK1/AKT Pathway. Reprod. Toxicol. 2022, 113, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.M.G.; da Silva, C.M.B.; Muñoz, P.M.; Rodríguez, A.M.; Dávila, M.P.; Rodríguez-Martínez, H.; Aparicio, I.M.; Tapia, J.A.; Ferrusola, C.O.; Peña, F.J. Phosphorylated AKT Preserves Stallion Sperm Viability and Motility by Inhibiting Caspases 3 and 7. Reproduction 2014, 148, 221–235. [Google Scholar] [CrossRef]
- Han, B.; Zhang, T.; Feng, Y.; Liu, X.; Zhang, L.; Chen, H.; Zeng, F.; Wang, M.; Liu, C.; Li, Y.; et al. Two Insulin Receptors Coordinate Oogenesis and Oviposition via Two Pathways in the Green Lacewing, Chrysopa pallens. J. Insect Physiol. 2020, 123, 104049. [Google Scholar] [CrossRef]
- Xu, K.-K.; Yan, Y.; Yan, S.-Y.; Xia, P.-L.; Yang, W.-J.; Li, C.; Yang, H. Disruption of the Serine/Threonine Kinase Akt Gene Affects Ovarian Development and Fecundity in the Cigarette Beetle, Lasioderma Serricorne. Front. Physiol. 2021, 12, 765819. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Huang, W.; Yue, W.; Li, D.; Zhi, J. Response of the Serine/Threonine Kinase AKT and Phosphoinositide-Dependent Kinase PDK in Frankliniella occidentalis (Thysanoptera: Thripidae) to Three Kinds of Foods and Their Regulation of Reproductive Function. Insect Mol. Biol. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-L.; Kesavapany, S.; Gravell, M.; Hamilton, R.S.; Schubert, M.; Amin, N.; Albers, W.; Grant, P.; Pant, H.C. A Cdk5 Inhibitory Peptide Reduces Tau Hyperphosphorylation and Apoptosis in Neurons. EMBO J. 2005, 24, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Rosales, J.L.; Lee, B.-C.; Chung, S.-H.; Fukui, Y.; Lee, N.-S.; Lee, K.-Y.; Jeong, Y.-G. Cdk5/P35 Expression in the Mouse Ovary. Mol. Cells 2004, 17, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rosales, J.L.; Lee, B.-C.; Modarressi, M.; Sarker, K.P.; Lee, K.-Y.; Jeong, Y.-G.; Oko, R.; Lee, K.-Y. Outer Dense Fibers Serve as a Functional Target for Cdk5·p35 in the Developing Sperm Tail. J. Biol. Chem. 2004, 279, 1224–1232. [Google Scholar] [CrossRef]
- Session, D.R.; Fautsch, M.P.; Avula, R.; Jones, W.R.; Nehra, A.; Wieben, E.D. Cyclin-Dependent Kinase 5 Is Expressed in both Sertoli Cells and Metaphase Spermatocytes. Fertil. Steril. 2001, 75, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, K.; Deng, Y.; He, R.; Zhang, X.; Zhong, G.; Hu, Q.; Weng, Q. Effects of 60Co-γ Radiation on Testis Physiological Aspects of Plutella xylostella (Linnaeus). Ecotoxicol. Environ. Saf. 2019, 169, 937–943. [Google Scholar] [CrossRef]
- Dobson, S.L. When More Is Less: Mosquito Population Suppression Using Sterile, Incompatible and Genetically Modified Male Mosquitoes. J. Med. Entomol. 2021, 58, 1980–1986. [Google Scholar] [CrossRef]
- Zhang, D.; Lees, R.S.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the Sterile Insect Technique with the Incompatible Insect Technique: III-Robust Mating Competitiveness of Irradiated Triple Wolbachia-Infected Aedes albopictus Males under Semi-Field Conditions. PLoS ONE 2016, 11, e0151864. [Google Scholar] [CrossRef]
- Zhang, D.; Lees, R.S.; Xi, Z.; Gilles, J.R.L.; Bourtzis, K. Combining the Sterile Insect Technique with Wolbachia-Based Approaches: II-A Safer Approach to Aedes albopictus Population Suppression Programmes, Designed to Minimize the Consequences of Inadvertent Female Release. PLoS ONE 2015, 10, e0135194. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Wang, M.; Zeng, Y.; He, F.; Li, S.; Zhang, K.; Weng, Q. Verification of AKT and CDK5 Gene and RNA Interference Combined with Irradiation to Mediate Fertility Changes in Plutella xylostella (Linnaeus). Int. J. Mol. Sci. 2024, 25, 4623. https://doi.org/10.3390/ijms25094623
Wen J, Wang M, Zeng Y, He F, Li S, Zhang K, Weng Q. Verification of AKT and CDK5 Gene and RNA Interference Combined with Irradiation to Mediate Fertility Changes in Plutella xylostella (Linnaeus). International Journal of Molecular Sciences. 2024; 25(9):4623. https://doi.org/10.3390/ijms25094623
Chicago/Turabian StyleWen, Jiaqi, Mengran Wang, Yuhao Zeng, Fengting He, Shifan Li, Ke Zhang, and Qunfang Weng. 2024. "Verification of AKT and CDK5 Gene and RNA Interference Combined with Irradiation to Mediate Fertility Changes in Plutella xylostella (Linnaeus)" International Journal of Molecular Sciences 25, no. 9: 4623. https://doi.org/10.3390/ijms25094623





