Biological Roles and Clinical Applications of Exosomes in Breast Cancer: A Brief Review
Abstract
:1. Introduction
2. Cancer-Derived Exosomes Can Advance Tumor Progression and Metastasis
3. Roles of Cancer-Derived Exosomes in the BC Microenvironment
4. Roles of Microenvironment-Derived Exosomes in BC
4.1. Effects of Fibroblast-Derived Exosomes
4.2. Effects of Immune Cell-Derived Exosomes
4.3. Effects of Adipocyte-Derived Exosomes
5. Roles of Exosomes in BC Drug Resistance
5.1. Exosomes in Chemoresistance
5.2. Exosomes in Hormone Resistance
5.3. Resistance of Exosomes to HER2-Targeted Therapy
6. Potential Clinical Applications of Exosomes in Treating BC
6.1. Exosomes as Potential Biomarkers of BC
6.2. Exosomes in BC Treatment
7. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| TNBC | Triple-negative breast cancer |
| ncRNAs | Noncoding RNAs |
| miRNAs | MicroRNAs |
| TME | Tumor microenvironment |
| CAFs | Cancer-associated fibroblasts |
| NFs | Normal fibroblasts |
| TAMs | Tumor-associated macrophages |
| CAAs | Cancer-associated adipocytes |
| FAO | Fatty acid oxidation |
| Erα | Estrogen receptor-α |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Hong, R.; Xu, B. Breast cancer: An up-to-date review and future perspectives. Cancer Commun. 2022, 42, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Goetz, M.P. Advances in systemic therapies for triple negative breast cancer. BMJ 2023, 381, e071674. [Google Scholar] [CrossRef]
- Cucciniello, L.; Gerratana, L.; Del Mastro, L.; Puglisi, F. Tailoring adjuvant endocrine therapy in early breast cancer: When, how, and how long? Cancer Treat. Rev. 2022, 110, 102445. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Papadaki, A.; Althobiti, M.; Alsaleem, M.; Aleskandarany, M.A.; Rakha, E.A. Breast cancer intratumour heterogeneity: Current status and clinical implications. Histopathology 2018, 73, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Vandenberghe, M.E.; Marchiò, C.; Ellis, I.O.; Sapino, A.; Rakha, E.A. Tumour Heterogeneity of Breast Cancer: From Morphology to Personalised Medicine. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2018, 85, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Kumar, H.; Gupta, N.V.; Jain, R.; Madhunapantula, S.V.; Babu, C.S.; Kesharwani, S.S.; Dey, S.; Jain, V. A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer. J. Adv. Res. 2023, 54, 271–292. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Z.; Hong, J.; Wu, J.; Huang, O.; He, J.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Targeting cancer-associated adipocyte-derived CXCL8 inhibits triple-negative breast cancer progression and enhances the efficacy of anti-PD-1 immunotherapy. Cell Death Dis. 2023, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.-S.; Kumar, A.; Vishakha; Behl, T.; Jha, S.K.; Tang, H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y.; Wang, R.; Cai, P.; Fang, Y. Design, synthesis, and anticancer activities of benzofuran–isatin hybrids tethered by pentylene and hexylene. J. Heterocycl. Chem. 2019, 56, 2052–2055. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, D.; Li, J.; Wang, Q.; Wo, L.; Zhang, X.; Hu, Z.; Wang, Z.; Zhan, M.; He, M. TGFB2-AS1 inhibits triple-negative breast cancer progression via interaction with SMARCA4 and regulating its targets TGFB2 and SOX2. Proc. Natl. Acad. Sci. USA 2022, 119, e2117988119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Sun, X.; Fang, Y.; Lu, S.S.; Ding, S.N.; Chen, X.S.; Shen, K.W. A Risk Stratification Model for Predicting Overall Survival and Surgical Benefit in Triple-Negative Breast Cancer Patients With de novo Distant Metastasis. Front. Oncol. 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.B.; Collie, S.P.; Parent, C.A. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2023, 34, 90–108. [Google Scholar] [CrossRef]
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021, 45, 1807–1831. [Google Scholar] [CrossRef]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2023, 10, 1894–1907. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Hánělová, K.; Raudenská, M.; Masařík, M.; Balvan, J. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun. Signal. CCS 2024, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Raimondo, S.; Chiesi, A.; Ciccia, F.; De Leo, G.; Alessandro, R. Exosomes as intercellular signaling organelles involved in health and disease: Basic science and clinical applications. Int. J. Mol. Sci. 2013, 14, 5338–5366. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta. Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; He, X.; Mei, C.; Ou, C. Exosome derived from tumor-associated macrophages: Biogenesis, functions, and therapeutic implications in human cancers. Biomark. Res. 2023, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Nail, H.M.; Chiu, C.C.; Leung, C.H.; Ahmed, M.M.M.; Wang, H.D. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J. Biomed. Sci. 2023, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Paul, S.; Ghosh, A.; Gupta, S.; Mukherjee, T.; Shankar, P.; Sharma, A.; Keshava, S.; Chauhan, S.C.; Kashyap, V.K.; et al. Extracellular Vesicles in Triple-Negative Breast Cancer: Immune Regulation, Biomarkers, and Immunotherapeutic Potential. Cancers 2023, 15, 4879. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, J.; Gu, J.; Shi, H.; Zhang, J.; Zhang, J.; Chen, Z.S.; Fang, X.; Zhu, T.; Zhang, X. Extracellular Vesicles in Cancer Drug Resistance: Roles, Mechanisms, and Implications. Adv. Sci. 2022, 9, e2201609. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Luo, L.; Hong, J.; Chen, X.; Shen, K.; Wang, Y.; Huang, R.; Wang, Z. An exosome-based specific transcriptomic signature for profiling regulation patterns and modifying tumor immune microenvironment infiltration in triple-negative breast cancer. Front. Immunol. 2023, 14, 1295558. [Google Scholar] [CrossRef] [PubMed]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, X.; Ni, J.; Zhang, H.; Duan, W.; Graham, P.; Li, Y. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020, 11, 987. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef]
- Deng, Z.; Cheng, Z.; Xiang, X.; Yan, J.; Zhuang, X.; Liu, C.; Jiang, H.; Ju, S.; Zhang, L.; Grizzle, W. Tumor cell cross talk with tumor-associated leukocytes leads to induction of tumor exosomal fibronectin and promotes tumor progression. Am. J. Pathol. 2012, 180, 390–398. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Li, J.; Shao, J.; Fang, L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem. Biophys. Res. Commun. 2018, 501, 486–493. [Google Scholar] [CrossRef]
- Ding, J.; Xu, Z.; Zhang, Y.; Tan, C.; Hu, W.; Wang, M.; Xu, Y.; Tang, J. Exosome-mediated miR-222 transferring: An insight into NF-κB-mediated breast cancer metastasis. Exp. Cell. Res. 2018, 369, 129–138. [Google Scholar]
- Wang, B.; Mao, J.-H.; Wang, B.-Y.; Wang, L.-X.; Wen, H.-Y.; Xu, L.-J.; Fu, J.-X.; Yang, H. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 2020, 489, 87–99. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, X.; Shao, C.; Fu, B.; Huang, Y.; Zhang, N.; Dou, X.; Zhang, Z.; Qiu, Y.; Wang, R. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021, 12, 38. [Google Scholar] [CrossRef]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Wang, D.; Yang, S.; Zhou, S.; Xu, H.; Zhang, H.; Zhong, S.; Feng, J. Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. J. Cell. Physiol. 2020, 235, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Duan, Z.; Zhang, C.; Wang, W.; He, H.; Liu, Y.; Wu, P.; Wang, S.; Song, M.; Chen, H. Mouse 4T1 breast cancer cell–derived exosomes induce proinflammatory cytokine production in macrophages via miR-183. J. Immunol. 2020, 205, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Lima, L.G.; Lobb, R.J.; Norris, E.L.; Hastie, M.L.; Krumeich, S.; Möller, A. Breast Cancer-Derived Exosomes Reflect the Cell-of-Origin Phenotype. Proteomics 2019, 19, e1800180. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.C.B.; Tofolo, M.V.; Nunes-Souza, E.; Marchi, R.; Okano, L.M.; Ruthes, M.; Rosolen, D.; Malheiros, D.; Fonseca, A.S.; Cavalli, L.R. Extracellular vesicles-associated miRNAs in triple-negative breast cancer: From tumor biology to clinical relevance. Life Sci. 2024, 336, 122332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, F.; Chen, J. The Role of Exosomal MicroRNAs in the Tumor Microenvironment of Breast Cancer. Int. J. Mol. Sci. 2019, 20, 3884. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Mayer, S.; Milo, T.; Isaacson, A.; Halperin, C.; Miyara, S.; Stein, Y.; Lior, C.; Pevsner-Fischer, M.; Tzahor, E.; Mayo, A.; et al. The tumor microenvironment shows a hierarchy of cell-cell interactions dominated by fibroblasts. Nat. Commun. 2023, 14, 5810. [Google Scholar] [CrossRef]
- Ma, C.; Yang, C.; Peng, A.; Sun, T.; Ji, X.; Mi, J.; Wei, L.; Shen, S.; Feng, Q. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol. Cancer 2023, 22, 170. [Google Scholar] [CrossRef]
- Arima, Y.; Matsueda, S.; Saya, H. Significance of Cancer-Associated Fibroblasts in the Interactions of Cancer Cells with the Tumor Microenvironment of Heterogeneous Tumor Tissue. Cancers 2023, 15, 2536. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.T.; Peng, B.; Zhang, D.X.; Ma, V.; Mathey-Andrews, C.A.; Lam, C.K.; Kiomourtzis, T.; Jin, J.; McReynolds, L.; Huang, L. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J. Extracell. Vesicles 2019, 8, 1599680. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.-H.; Fadare, O.; Pizzo, D.P. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat. Cell Biol. 2018, 20, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kwon, A.; Huh, Y.H.; Rhee, S.; Song, W.K. Tumor-derived miR-130b-3p induces cancer-associated fibroblast activation by targeting SPIN90 in luminal A breast cancer. Oncogenesis 2022, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhong, J.; Li, M.; Zhou, Y.; Lin, Q.; Zong, S.; Luo, W.; Wang, J.; Wang, K. Tumor-derived Cav-1 promotes pre-metastatic niche formation and lung metastasis in breast cancer. Theranostics 2023, 13, 1684. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, T.; Chen, J.; Ni, H.; Li, W. Survivin in breast cancer–derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 2020, 295, 13737–13752. [Google Scholar] [CrossRef]
- Ji, X.; Huang, X.; Li, C.; Guan, N.; Pan, T.; Dong, J.; Li, L. Effect of tumor-associated macrophages on the pyroptosis of breast cancer tumor cells. Cell Commun. Signal. CCS 2023, 21, 197. [Google Scholar] [CrossRef]
- Song, J.; Xiao, T.; Li, M.; Jia, Q. Tumor-associated macrophages: Potential therapeutic targets and diagnostic markers in cancer. Pathol. Res. Pract. 2023, 249, 154739. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bao, X.; Zou, Y.; Wang, L.; Li, Y.; Yang, L.; Liao, A.; Zhang, X.; Jiang, X.; Liang, D.; et al. d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci. Adv. 2023, 9, eadg2697. [Google Scholar] [CrossRef] [PubMed]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-associated macrophages: An effective player of the tumor microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, R.; Lu, Z.; Wang, Z.; Chen, X.; Huang, D. Exosomes from M1-polarized macrophages promote apoptosis in lung adenocarcinoma via the miR-181a-5p/ETS1/STK16 axis. Cancer Sci 2022, 113, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Lima, L.G.; Chai, E.P.Z.; Muller, A.; Lobb, R.J.; Krumeich, S.; Wen, S.W.; Wiegmans, A.P.; Möller, A. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front. Immunol. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Xun, J.; Du, L.; Gao, R.; Shen, L.; Wang, D.; Kang, L.; Zhang, Z.; Zhang, Y.; Yue, S.; Feng, S. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 2021, 11, 6847. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Mo, Z.; Lai, G.; Chen, X.; Li, R.; Wu, R.; Zhu, J.; Zheng, F. Targeting tumor exosomal circular RNA cSERPINE2 suppresses breast cancer progression by modulating MALT1-NF-κB-IL-6 axis of tumor-associated macrophages. J. Exp. Clin. Cancer Res. 2023, 42, 48. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shi, W.; Hu, W.; Zhao, Y.; Zhao, X.; Dong, F.; Xin, Y.; Peng, T.; Liu, C. Endoplasmic reticulum stress promotes breast cancer cells to release exosomes circ_0001142 and induces M2 polarization of macrophages to regulate tumor progression. Pharmacol. Res. 2022, 177, 106098. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell. Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef]
- Frank, A.-C.; Ebersberger, S.; Fink, A.F.; Lampe, S.; Weigert, A.; Schmid, T.; Ebersberger, I.; Syed, S.N.; Brüne, B. Apoptotic tumor cell-derived microRNA-375 uses CD36 to alter the tumor-associated macrophage phenotype. Nat. Commun. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Rabe, D.C.; Walker, N.D.; Rustandy, F.D.; Wallace, J.; Lee, J.; Stott, S.L.; Rosner, M.R. Tumor extracellular vesicles regulate macrophage-driven metastasis through CCL5. Cancers 2021, 13, 3459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, K.; Sharma, S.; Xing, F.; Wu, S.-Y.; Tyagi, A.; Deshpande, R.; Singh, R.; Wabitsch, M.; Mo, Y.-Y. Exosomal miR-1304-3p promotes breast cancer progression in African Americans by activating cancer-associated adipocytes. Nat. Commun. 2022, 13, 7734. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Z.; Yao, F.; Sun, K.; Li, Z.; Sun, S.; Li, C. Breast cancer cell-derived exosome-delivered microRNA-155 targets UBQLN1 in adipocytes and facilitates cancer cachexia-related fat loss. Hum. Mol. Genet. 2023, 32, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Feng, J.; Lyu, F.; Xing, F.; Sharma, S.; Liu, Y.; Wu, S.-Y.; Zhao, D.; Tyagi, A.; Deshpande, R.P. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 2021, 12, 5196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.W.; Chen, Y.; Li, J.Y.; Xiao, L.; Zhang, W.J.; Zhao, J.L.; Gu, H.C.; Wu, H.Y.; Zuo, G.S.L.; Deng, K.Y. Bone marrow cells are differentiated into MDSCs by BCC-Ex through down-regulating the expression of CXCR4 and activating STAT3 signalling pathway. J. Cell. Mol. Med. 2021, 25, 5497–5510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, H.; Wang, J.; Li, L.; Chen, A.; Li, Z. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. Nucleic Acids 2020, 19, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Li, H.; Ma, X.; Ma, Y.; He, J.; Gao, Y.; Li, J. Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Ther. 2021, 28, 634–648. [Google Scholar] [CrossRef]
- Yan, Z.; Sheng, Z.; Zheng, Y.; Feng, R.; Xiao, Q.; Shi, L.; Li, H.; Yin, C.; Luo, H.; Hao, C. Cancer-associated fibroblast-derived exosomal miR-18b promotes breast cancer invasion and metastasis by regulating TCEAL7. Cell Death Dis. 2021, 12, 1120. [Google Scholar] [CrossRef]
- Dou, D.; Ren, X.; Han, M.; Xu, X.; Ge, X.; Gu, Y.; Wang, X. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front. Immunol. 2020, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sang, Y.; Song, X.; Zhang, D.; Wang, L.; Zhao, W.; Liang, Y.; Zhang, N.; Yang, Q. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics 2021, 11, 3932. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, C.; Zhan, Y.; Wang, H.; Jiang, X.; Li, W. Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene 2017, 36, 4692–4705. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Principe, S.; Jackson, H.W.; Luga, V.; Fang, H.; Molyneux, S.D.; Shao, Y.W.; Aiken, A.; Waterhouse, P.D.; Karamboulas, C. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat. Cell Biol. 2014, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Peng, M.; Liu, S.; Liu, Y.; Wan, X.; Hou, Y.; Qin, Y.; Yang, L.; Chen, S.; Zeng, H. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion. J. Extracell. Vesicles 2021, 10, e12146. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fan, P.; Zhang, C.; Xie, J.; Gu, X.; Lei, S.; Chen, Z.; Huang, Z. Exosomal microRNA-503-3p derived from macrophages represses glycolysis and promotes mitochondrial oxidative phosphorylation in breast cancer cells by elevating DACT2. Cell Death Discov. 2021, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, C.; Guo, J.; Wang, W.; Si, Q.; Chen, C.; Luo, Y.; Duan, Z. Exosomal miRNA-223-3p derived from tumor associated macrophages promotes pulmonary metastasis of breast cancer 4T1 cells. Transl. Oncol. 2023, 35, 101715. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Q.; Zhang, X.; Han, Q.; Li, H.; Mao, Y.; Wang, X.; Guo, H.; Irwin, D.M.; Niu, G. Exosomes from macrophages exposed to apoptotic breast cancer cells promote breast cancer proliferation and metastasis. J. Cancer 2019, 10, 2892. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhu, W.W.; Wang, Z.; Huang, J.B.; Wang, S.H.; Bai, F.M.; Li, T.E.; Zhu, Y.; Zhao, J.; Yang, X.; et al. Driver mutations of intrahepatic cholangiocarcinoma shape clinically relevant genomic clusters with distinct molecular features and therapeutic vulnerabilities. Theranostics 2022, 12, 260–276. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Li, Z.; Li, S.; Wen, Z.; Cao, L.; Chen, Y.; Xue, P.; Li, H.; Zhang, D. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022, 13, 94. [Google Scholar] [CrossRef]
- Guan, H.; Peng, R.; Fang, F.; Mao, L.; Chen, Z.; Yang, S.; Dai, C.; Wu, H.; Wang, C.; Feng, N. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020, 235, 9729–9742. [Google Scholar] [CrossRef]
- Wang, S.; Su, X.; Xu, M.; Xiao, X.; Li, X.; Li, H.; Keating, A.; Zhao, R.C. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal. 2021, 14, eabj2807. [Google Scholar] [CrossRef]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget 2017, 8, 81880. [Google Scholar] [CrossRef] [PubMed]
- Shedden, K.; Xie, X.T.; Chandaroy, P.; Chang, Y.T.; Rosania, G.R. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003, 63, 4331–4337. [Google Scholar]
- Lv, M.-M.; Zhu, X.-Y.; Chen, W.-X.; Zhong, S.-L.; Hu, Q.; Ma, T.-F.; Zhang, J.; Chen, L.; Tang, J.-H.; Zhao, J.-H. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumor Biol. 2014, 35, 10773–10779. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wang, T.; Sun, X.; Zhang, P.; Chen, Y.; Jin, J.; Hua, D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J. Surg. Oncol. 2017, 115, 932–940. [Google Scholar] [CrossRef]
- Kreger, B.T.; Johansen, E.R.; Cerione, R.A.; Antonyak, M.A. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers 2016, 8, 111. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Ye, M.; Wu, J.; Ma, L.; Chen, H. Cisplatin-resistant MDA-MB-231 cell-derived exosomes increase the resistance of recipient cells in an exosomal miR-423-5p-dependent manner. Curr. Drug Metab. 2019, 20, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, X.; Guo, G.; Qian, X.; Dou, D.; Zhang, Z.; Xu, X.; Duan, X. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J. Cell. Physiol. 2020, 235, 6896–6904. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ren, Z.J.; Tang, J.H.; Yu, Q. Exosomal MicroRNA MiR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer. Cell. Physiol. Biochem. 2018, 44, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Semina, S.E.; Scherbakov, A.M.; Vnukova, A.A.; Bagrov, D.V.; Evtushenko, E.G.; Safronova, V.M.; Golovina, D.A.; Lyubchenko, L.N.; Gudkova, M.V.; Krasil’nikov, M.A. Exosome-mediated transfer of cancer cell resistance to antiestrogen drugs. Molecules 2018, 23, 829. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Yang, M.F.; Ren, Y.Q.; Wu, C.H.; Wang, L.Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4362–4368. [Google Scholar] [PubMed]
- Wei, Y.; Lai, X.; Yu, S.; Chen, S.; Ma, Y.; Zhang, Y.; Li, H.; Zhu, X.; Yao, L.; Zhang, J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 2014, 147, 423–431. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Chen, R.; Zhang, Y.; Zou, K.; Ye, M.; He, X.; Zhang, F.; Han, J. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int. J. Oncol. 2018, 53, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, M.; Xing, P.; Yan, X.; Xie, B. Increased expression of exosomal AGAP2-AS1 (AGAP2 antisense RNA 1) in breast cancer cells inhibits trastuzumab-induced cell cytotoxicity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2211. [Google Scholar] [CrossRef]
- Martinez, V.G.; O’Neill, S.; Salimu, J.; Breslin, S.; Clayton, A.; Crown, J.; O’Driscoll, L. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology 2017, 6, e1362530. [Google Scholar] [CrossRef]
- Han, M.; Hu, J.; Lu, P.; Cao, H.; Yu, C.; Li, X.; Qian, X.; Yang, X.; Yang, Y.; Han, N. Exosome-transmitted miR-567 reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell Death Dis. 2020, 11, 43. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, W.; Bu, J.; Li, Y.; Li, R.; Nie, R.; Xiao, C.; Ma, K.; Huang, X.; Li, Y. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol. Carcinog. 2019, 58, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.M.; Walsh, P.T. Chronic obstructive pulmonary disease: Evidence for an autoimmune component. Cell. Mol. Immunol. 2009, 6, 81–86. [Google Scholar] [CrossRef]
- Golubovskaya, V.M. Focal adhesion kinase and cross-linked signaling in cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.-Y.; Li, M.-X.; Pan, W.-L.; Chen, Y.; Li, M.-M.; Pang, J.-X.; Zheng, L.; Chen, J.-X.; Duan, W.-J. In situ detection of plasma exosomal microRNA-1246 for breast cancer diagnostics by a Au nanoflare probe. ACS Appl. Mater. Interfaces 2018, 10, 39478–39486. [Google Scholar] [CrossRef] [PubMed]
- Stevic, I.; Müller, V.; Weber, K.; Fasching, P.A.; Karn, T.; Marmé, F.; Schem, C.; Stickeler, E.; Denkert, C.; van Mackelenbergh, M. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, Y.; Guo, R.; Zhao, J.; Chi, W.; Lai, H.; Wang, J.; Wang, Z.; Li, L.; Sang, Y. Plasma extracellular vesicle long RNA profiles in the diagnosis and prediction of treatment response for breast cancer. NPJ Breast Cancer 2021, 7, 154. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Wang, R. Current perspectives on naturally occurring saponins as anticancer agents. Arch. Pharm. 2022, 355, e2100469. [Google Scholar] [CrossRef]
- Herdiana, Y.; Husni, P.; Nurhasanah, S.; Shamsuddin, S.; Wathoni, N. Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy. Polymers 2023, 15, 2953. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Feng, C.; Zhang, J.; Qin, Y.; Meng, L. Advances in the Pharmacological Activities and Effects of Perilla Ketone and Isoegomaketone. Evid. Based Complement. Altern. Med. Ecam 2022, 2022, 8809792. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, Z.; Han, X.; Zhen, L.; Luo, C.; Liu, M.; Yu, K.; Ren, Y. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics 2019, 9, 2618. [Google Scholar] [CrossRef]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.-E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-derived extracellular vesicles breach the intact blood–brain barrier via transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef]
- Park, E.J.; Jung, H.J.; Choi, H.J.; Jang, H.J.; Park, H.J.; Nejsum, L.N.; Kwon, T.H. Exosomes co-expressing AQP5-targeting miRNAs and IL-4 receptor-binding peptide inhibit the migration of human breast cancer cells. FASEB J. 2020, 34, 3379–3398. [Google Scholar] [CrossRef]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.-J.; Zhang, Y. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, Y.; Yang, R.; Liu, C.; Hsu, J.-M.; Jiang, Z.; Sun, L.; Wei, Y.; Li, C.-W.; Yu, D. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene 2021, 40, 4992–5001. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Ye, T.; Wang, J.; Lyu, C.; Qing, S.; Ding, Z.; Gao, X.; Jia, R.; Yu, D. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci. Transl. Med. 2021, 13, eabb6981. [Google Scholar] [CrossRef] [PubMed]

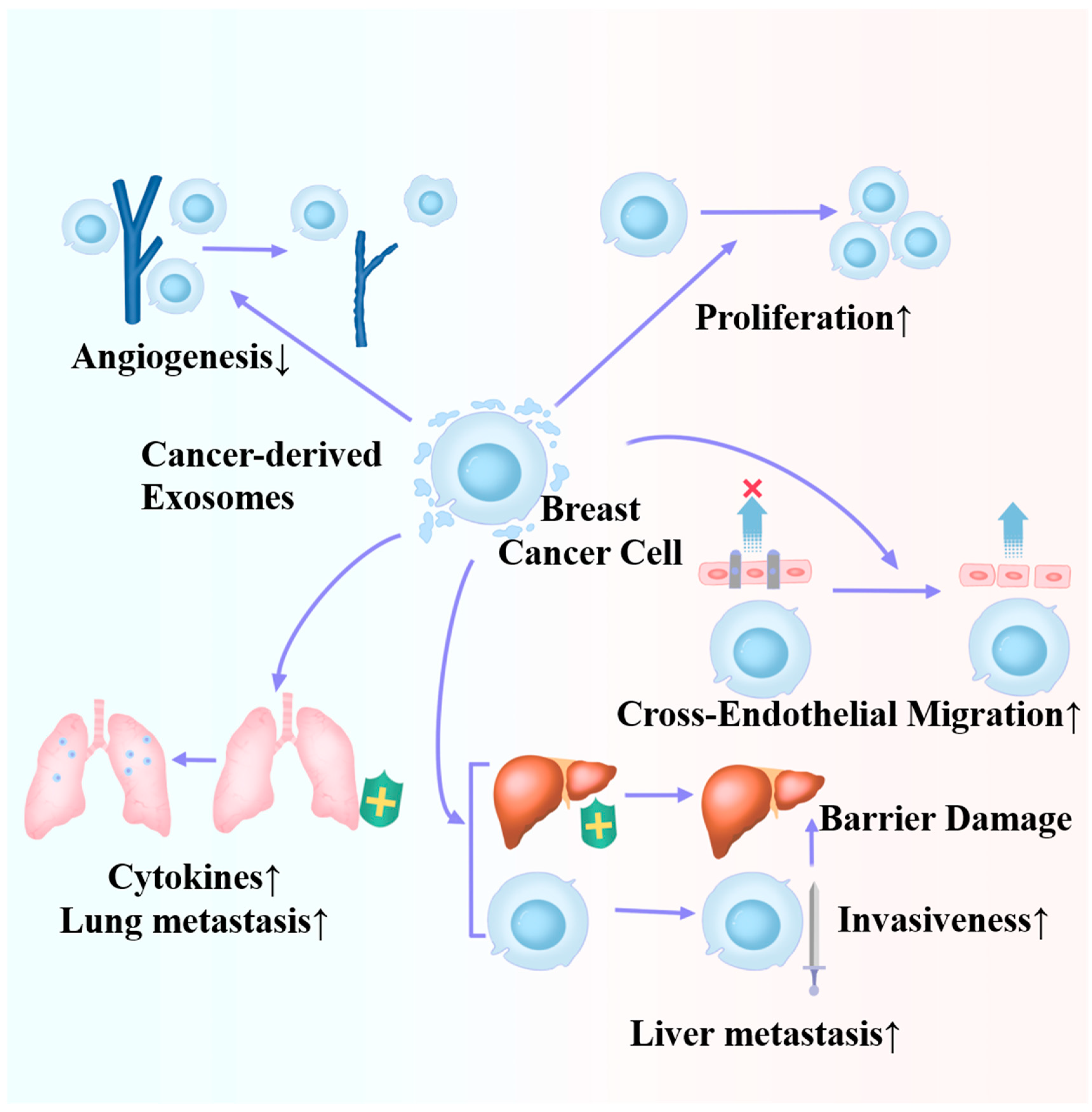


| Biological Roles | Intermediates | Major Mechanisms | References |
|---|---|---|---|
| BC metastasis | / | FAK/Src-dependent proteins | [37] |
| BC progression | miR-130a-3p | RAB5B | [38] |
| BC invasiveness and metastasis | miR-222 | PDZ/PDLIM2/NF-kB | [39] |
| BC progression and metastasis | miR-1910-3p | MTMR3/NF-kB | [40] |
| HUVEC angiogenesis (BC inhibition) | miR-145 | IRS1/Raf/ERK IRS1/PI3K/Akt/mTOR | [41] |
| BC cross-endothelial migration | miR-939 | Vascular endothelial cadherin | [42] |
| BC liver metastasis | miR-4443 | Barrier damage TIMP2/MMP-2 | [43] |
| BC lung metastasis | miR-183-5p | PPP1CA-IL2/TNFα | [44] |
| TME Cell Subtype | Biological Roles | Intermediates | Major Mechanisms | References |
|---|---|---|---|---|
| Cancer-associated fibroblasts | ||||
| NF to CAF | miR-9 | / | [55] | |
| NF to CAF | miR-125b | / | [56] | |
| BC tumor growth | miR-105 | Metabolism reprogramming | [57] | |
| BC progression | miR-130b-3p | SPIN90 | [58] | |
| BC lung metastasis | Cav-1 | Extracellular matrix deposition | [59] | |
| BC metastasis and proliferation | Survivin | SOD-1 | [60] | |
| Tumor-associated macrophages | ||||
| M1 to M2 | GP130 | STAT3 pathway | [66] | |
| M1 to M2 | miR-138-5p | KDM6B | [67] | |
| BC lung metastasis | Lin28B | PD-L1/Cytokine imbalance | [68] | |
| M1 to M2 | Circ_0001142 | Endoplasmic reticulum | [70] | |
| BC immune escape | miR-27a-3p | PD-L1 | [71] | |
| BC migration and infiltration | CD375/miR-63 | TNS3PXN | [72] | |
| BC lung metastasis | CCL5 | Immune infiltration | [73] | |
| Cancer-related adipocytes | ||||
| BC progression | miR-1304-3p | GATA2 | [74] | |
| BC inhibition | miR-155 | White adipose tissue browning | [75] | |
| BC progression | miR-122 | Pyruvate kinase | [76] |
| Tumor Microenvironment-Derived Exosomes | Biological Roles | Intermediates | Major Mechanisms | References |
|---|---|---|---|---|
| Fibroblast-derived exosomes | ||||
| BC invasiveness | miR-181d-5p | CDX2/HOXA5 axis | [79] | |
| BC metastasis and invasion | miR-1b/miR-3-7p | TCEAL1 GLIS232 | [80] | |
| BC metastasis and invasion | miR-18b | TCEAL7/NF-kB/Snail/EMT | [81] | |
| BC proliferation and migration | miR-92 | PD-L1/LATS2 | [82] | |
| BC tumor-infiltrating immune cell function | ||||
| BC proliferation | miR-500a-5p | USP28 | [83] | |
| BC metastasis | Wnt10b parasecretion | [84] | ||
| BC progression | ADAM10 | RhoA/Notch axis | [85] | |
| BC invasiveness | GPR64 | IL-8/MMP9 | [86] | |
| Immune cell-derived exosomes | ||||
| BC progression | miR-503-3p | DACT2/Wnt/β-Catenin | [87] | |
| BC lung metastasis | miR-223-3p | Cbx5 | [88] | |
| BC recurrence and metastasis after chemotherapy | / | STAT3 | [89] | |
| Cancer progression | Circ_0020256 | miR-432-5p/E2F3 | [91] | |
| Cancer progression | miR-95 | JunB/EMT | [92] | |
| Adipocyte-derived exosomes | ||||
| BC cell proliferation and migration | / | Hippo | [93] | |
| BC cancer stem cell | TSP5 COMP/BRD2 or BRD3 | EMT | [94] | |
| BC differentiation, migration, and stemness | miR-140 | SOX2/SOX9 | [95] | |
| Cancer invasiveness and migration | Proteins involved in fatty acid oxidation | Metabolic reprogramming | [96] | |
| Cancer invasiveness | MMP3 | MMP9 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, R.; Shen, K.; Huang, R.; Wang, Z. Biological Roles and Clinical Applications of Exosomes in Breast Cancer: A Brief Review. Int. J. Mol. Sci. 2024, 25, 4620. https://doi.org/10.3390/ijms25094620
Wang H, Wang R, Shen K, Huang R, Wang Z. Biological Roles and Clinical Applications of Exosomes in Breast Cancer: A Brief Review. International Journal of Molecular Sciences. 2024; 25(9):4620. https://doi.org/10.3390/ijms25094620
Chicago/Turabian StyleWang, Han, Ruo Wang, Kunwei Shen, Renhong Huang, and Zheng Wang. 2024. "Biological Roles and Clinical Applications of Exosomes in Breast Cancer: A Brief Review" International Journal of Molecular Sciences 25, no. 9: 4620. https://doi.org/10.3390/ijms25094620





