Dolutegravir and Folic Acid Interaction during Neural System Development in Zebrafish Embryos
Abstract
:1. Introduction
2. Results
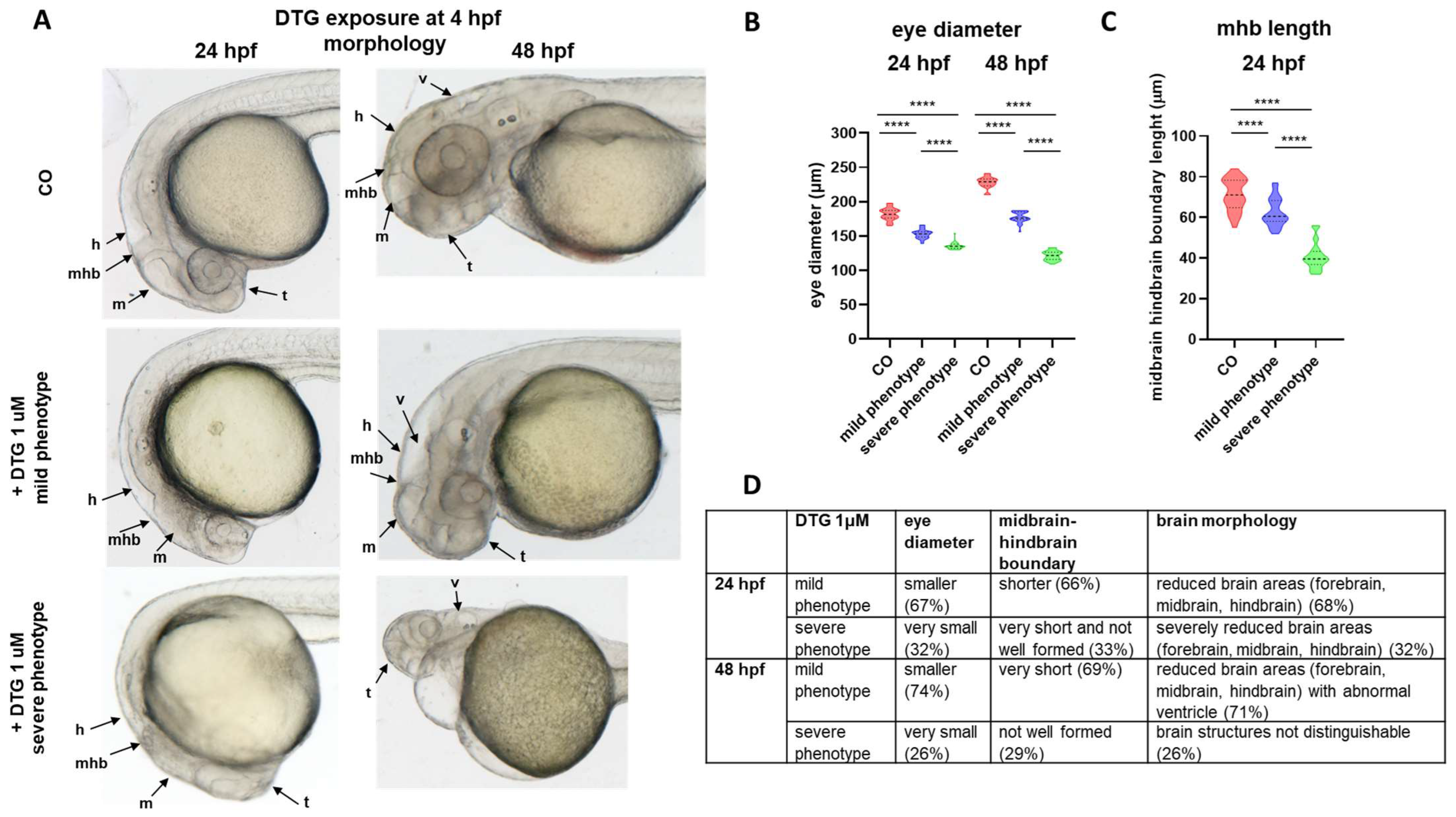
2.1. Early Embryonic Exposure to Dolutegravir Induces Morphological Malformations Particularly Evident in the Developing Brain Region
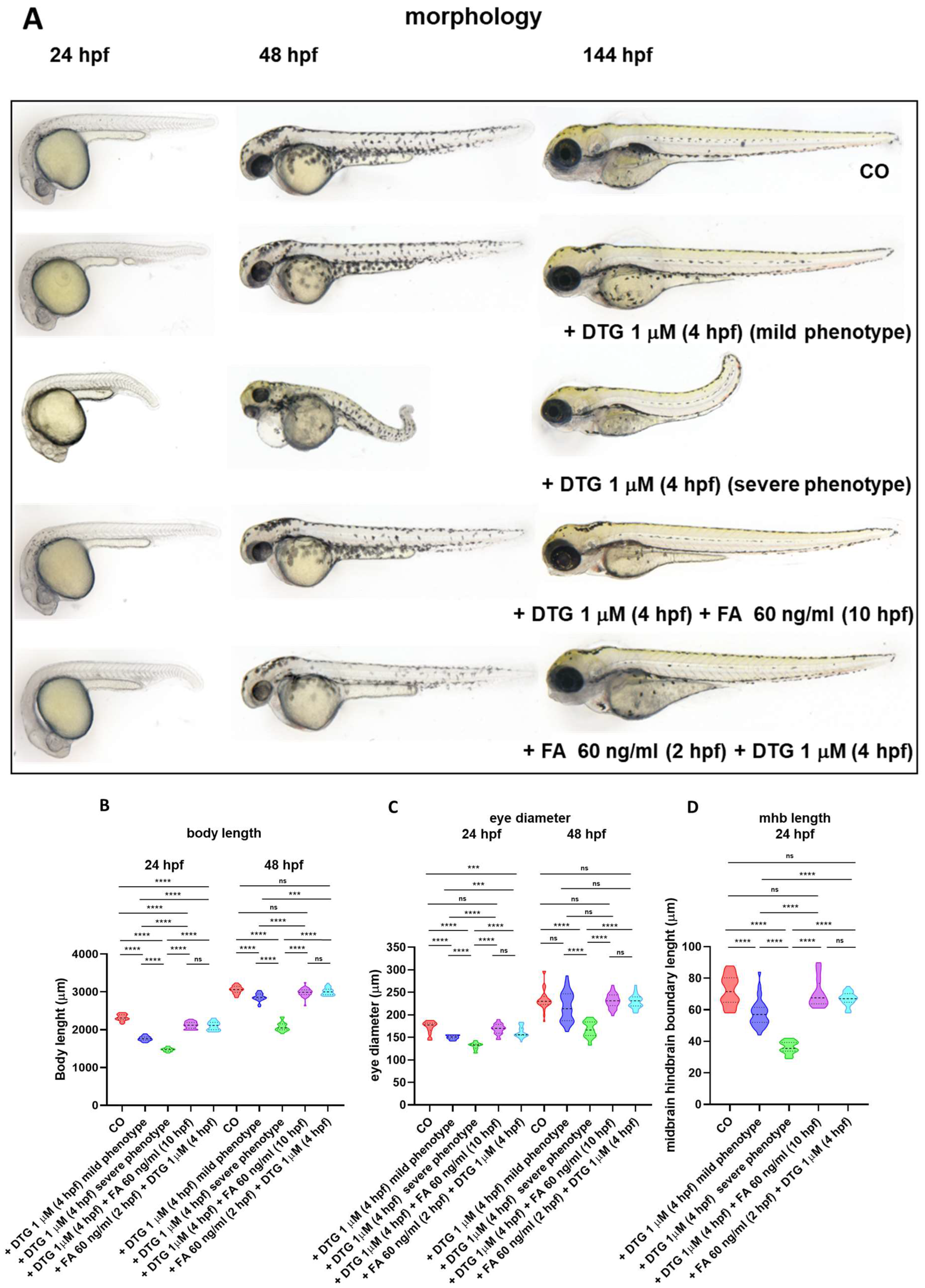
2.2. Gross Morphological Malformations Induced by Early Embryonic Exposure of Zebrafish Embryos to Dolutegravir Are Prevented by Pre-Exposure and Rescued by Post-Exposure to Folic Acid
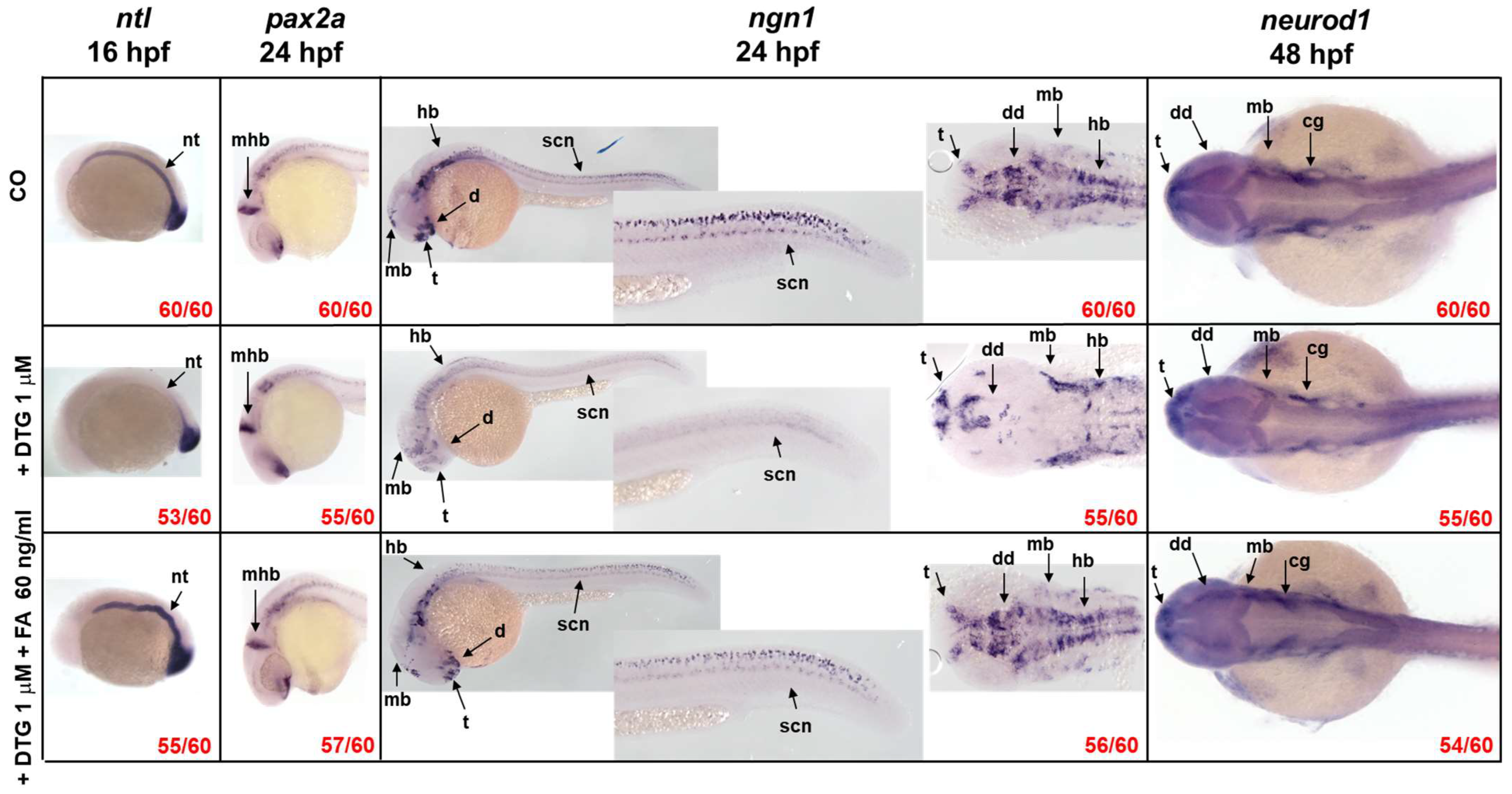
2.3. DTG Exposure Induces Neurodevelopmental Defects over Time That Are Rescued by Post-Exposure Folate Supplementation
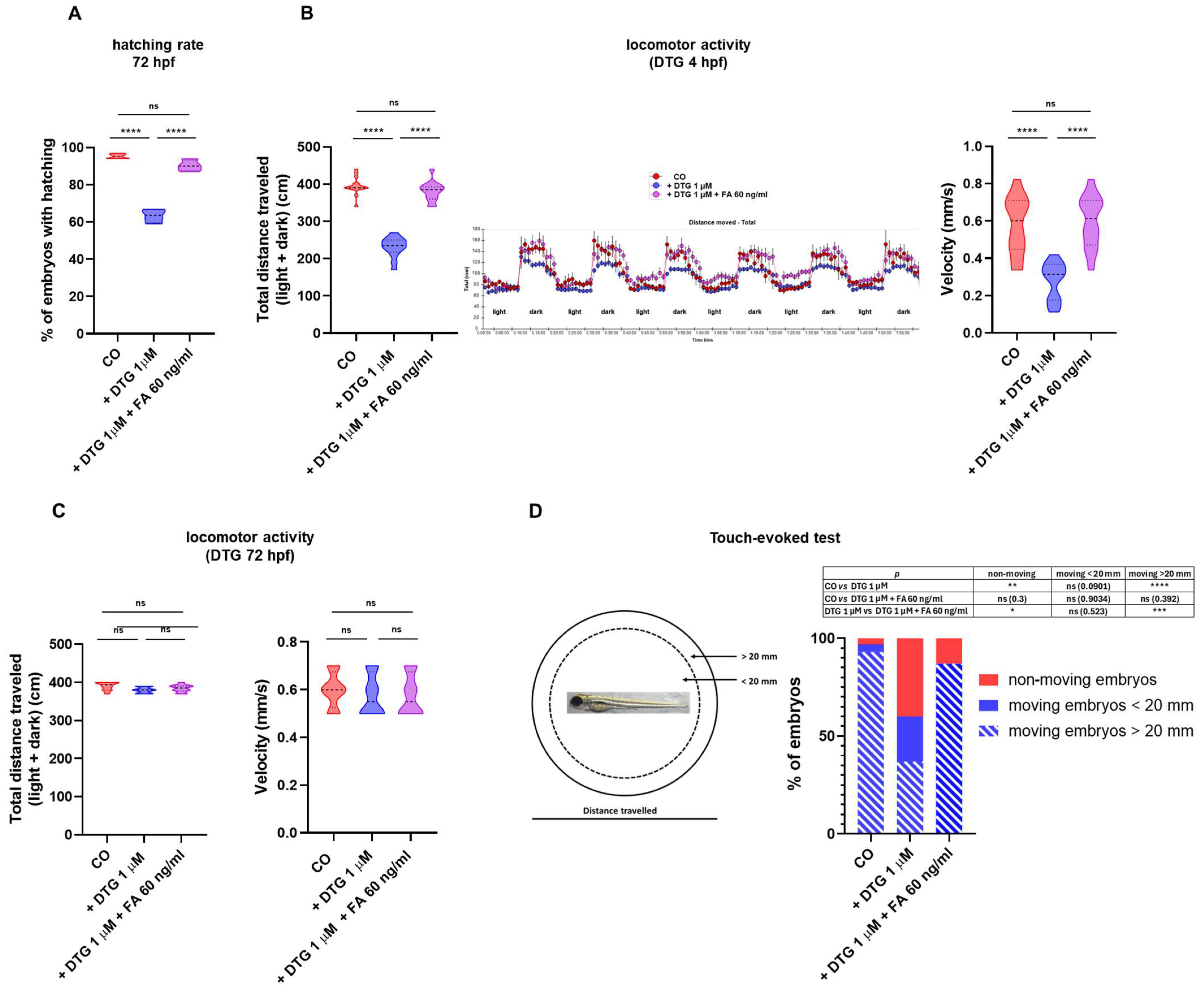
2.4. DTG Exposure Results in Decreased Hatching Rate, Locomotor Activity and Touch-Evoked Swimming That Are Rescued by Folate Supplementation
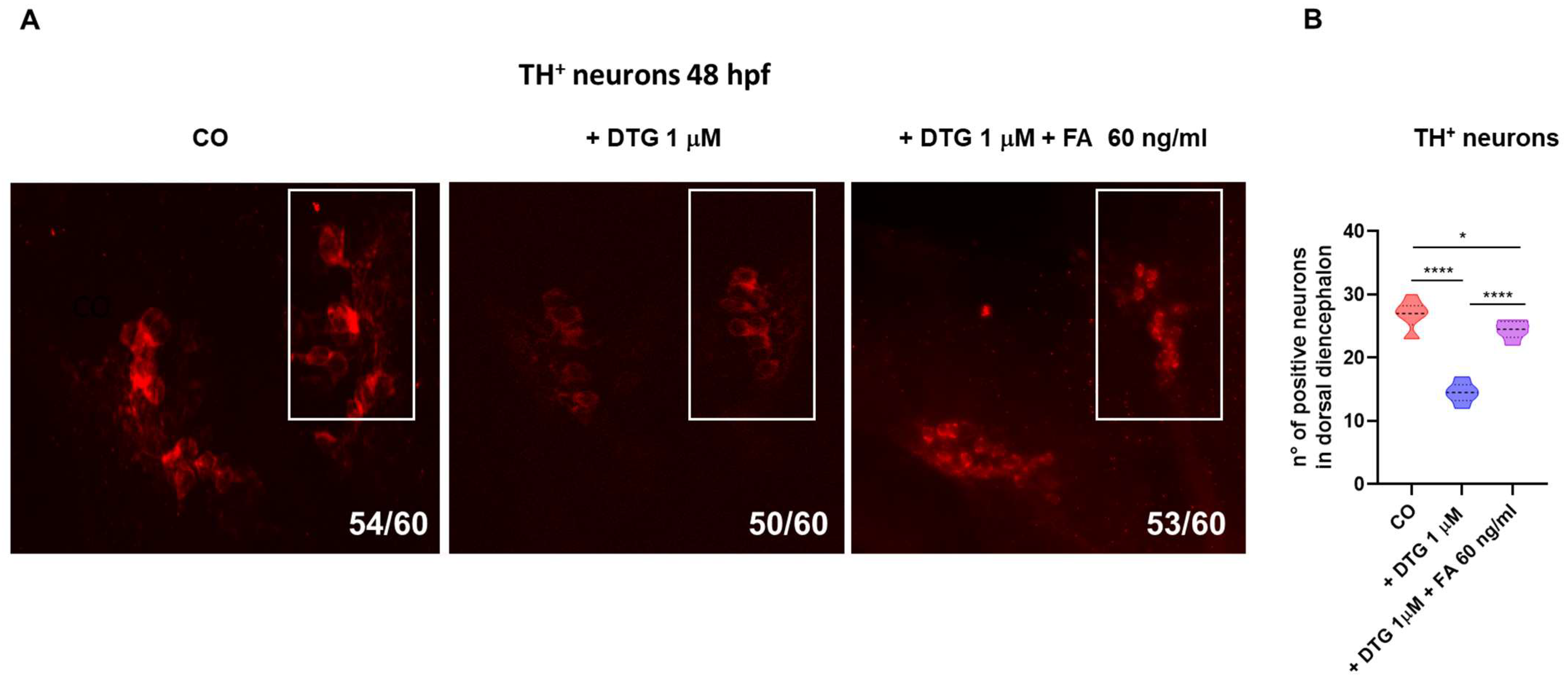
2.5. DTG Exposure Decreases the Number of Dopaminergic Neurons in the Dorsal Diencephalon While Folate Supplementation Rescued This Loss
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Fish Maintenance and Collection of Eggs
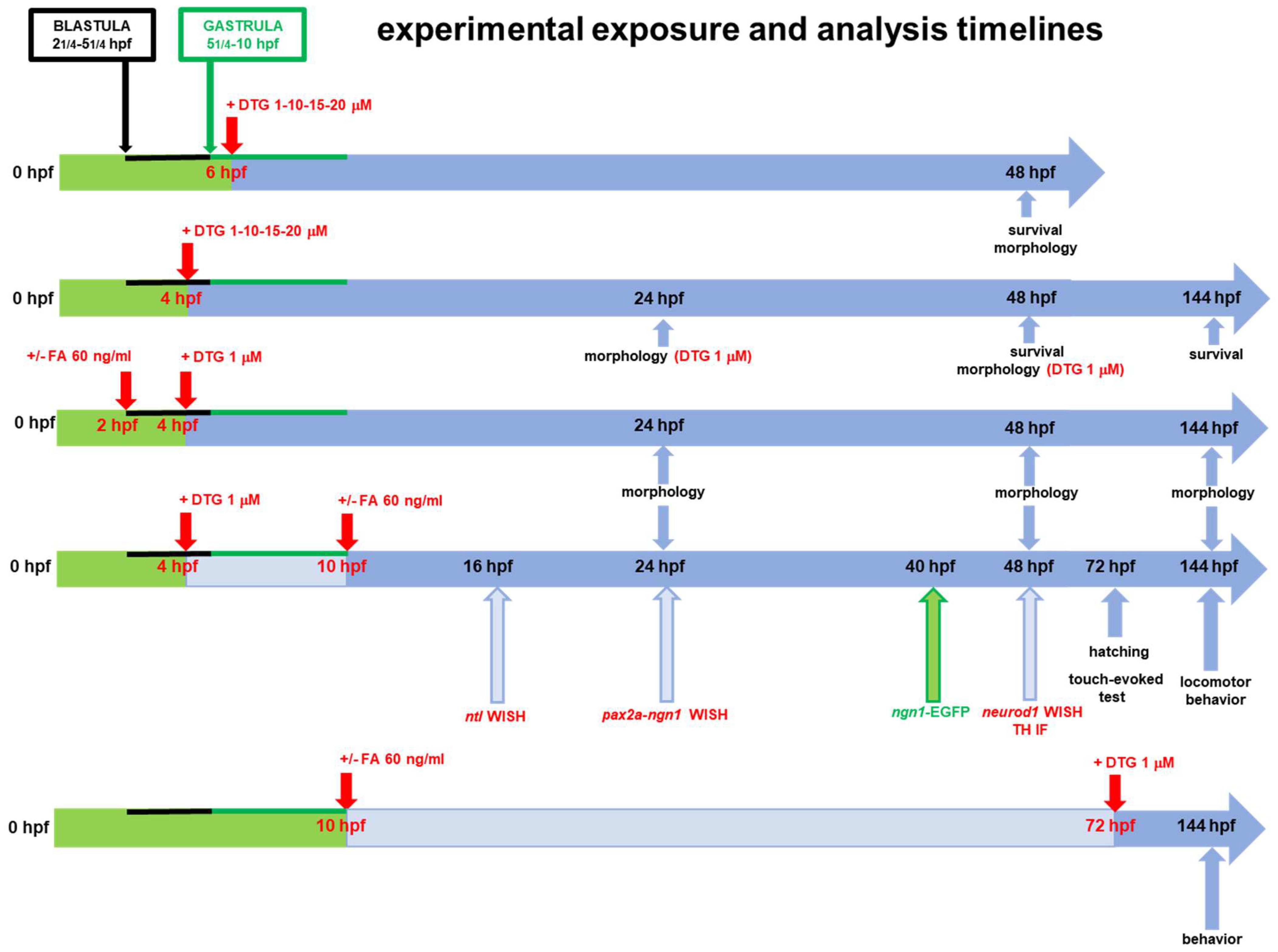
4.3. Drug Exposure of Embryos
4.4. Evaluation of Mortality and Gross Developmental Morphology
4.5. Whole-Mount In Situ Hybridization (WISH)
4.6. Image Analysis of Neurogenin 1 (ngn1) Expression with Tg(ngn1:EGFP) Line
4.7. Hatching Rate
4.8. Behavior Assessment by the Light–Dark Locomotion and Touch-Evoked Tests
4.9. Tyrosine Hydroxylase Immunofluorescence Experiments
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef] [PubMed]
- Teira, R.; Diaz-Cuervo, H.; Aragão, F.; Marguet, S.; de la Fuente, B.; Muñoz, M.J.; Abdulghani, N.; Ribera, E.; Domingo, P.; Deig, E.; et al. Real world effectiveness of standard of care triple therapy versus two-drug combinations for treatment of people living with HIV. PLoS ONE 2021, 16, e0249515. [Google Scholar] [CrossRef] [PubMed]
- Ciccullo, A.; Baldin, G.; Borghi, V.; Lagi, F.; Latini, A.; D’ettorre, G.; Oreni, L.; Fusco, P.; Capetti, A.; Fabbiani, M.; et al. Real-Life Impact of Drug Toxicity on Dolutegravir Tolerability: Clinical Practice Data from a Multicenter Italian Cohort. Viruses 2022, 14, 163. [Google Scholar] [CrossRef]
- Zash, R.; Makhema, J.; Shapiro, R.L. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N. Engl. J. Med. 2018, 379, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Zash, R.; Holmes, L.; Diseko, M.; Jacobson, D.L.; Brummel, S.; Mayondi, G.; Isaacson, A.; Davey, S.; Mabuta, J.; Mmalane, M.; et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N. Engl. J. Med. 2019, 381, 827–840. [Google Scholar] [CrossRef]
- Pereira, G.F.M.; Kim, A.; Jalil, E.M.; Fonseca, F.F.; E Shepherd, B.; Veloso, V.G.; Rick, F.; Ribeiro, R.; Pimenta, M.C.; Beber, A.; et al. Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: A retrospective national cohort study. Lancet HIV 2021, 8, e33–e41. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Zhu, W.; Lampe, M.A.; Huang, Y.A.; Hoover, K.W. Dolutegravir and pregnancy outcomes including neural tube defects in the USA during 2008–2020: A national cohort study. Lancet HIV 2023, 10, e588–e596. [Google Scholar] [CrossRef] [PubMed]
- Crowell, C.S.; Williams, P.L.; Yildirim, C.; Van Dyke, R.B.; Smith, R.; Chadwick, E.G.; Seage, G.R.; Diperna, A.; Hazra, R. Safety of in-utero antiretroviral exposure: Neurologic outcomes in children who are HIV-exposed but uninfected. AIDS 2020, 34, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- NEJM Journal Watch. 2021. Available online: https://www.jwatch.org/na53453/2021/04/23/antiretroviral-therapy-pregnant-women-with-hiv-safe-and (accessed on 13 February 2024).
- Cabrera, R.M.; Souder, J.P.; Steele, J.W.; Yeo, L.; Tukeman, G.; Gorelick, D.A.; Finnell, R.H. The antagonism of folate receptor by dolutegravir: Developmental toxicity reduction by supplemental folic acid. AIDS 2019, 33, 1967–1976. [Google Scholar] [CrossRef]
- Gilmore, J.C.; Hoque, T.; Dai, W.; Mohan, H.; Dunk, C.; Serghides, L.; Bendayan, R. Interaction between dolutegravir and folate transporters and receptor in human and rodent placenta. EBioMedicine 2021, 75, 103771. [Google Scholar] [CrossRef]
- Mohan, H.; Nguyen, J.; MacKenzie, B.; Yee, A.; Laurette, E.Y.; Sanghvi, T.; Tejada, O.; Dontsova, V.; Leung, K.-Y.; Goddard, C.; et al. Folate deficiency increases the incidence of dolutegravir-associated foetal defects in a mouse pregnancy model. eBioMedicine 2023, 95, 104762. [Google Scholar] [CrossRef] [PubMed]
- Small, C.D.; Crawford, B.D. Matrix metalloproteinases in neural development: A phylogenetically diverse perspective. Neural Regen. Res. 2016, 11, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bade, A.N.; McMillan, J.M.; Liu, Y.; Edagwa, B.J.; Gendelman, H.E. Dolutegravir Inhibition of Matrix Metalloproteinases Affects Mouse Neurodevelopment. Mol. Neurobiol. 2021, 58, 5703–5721. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish-from embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Schnoll, J.G.; Temsamrit, B.; Zhang, D.; Song, H.; Ming, G.L.; Christian, K.M. Evaluating Neurodevelopmental Consequences of Perinatal Exposure to Antiretroviral Drugs: Current Challenges and New Approaches. J. Neuroimmune Pharmacol. 2021, 16, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.G.; Gendelman, H.E.; Bade, A.N. HIV-1 Integrase Strand Transfer Inhibitors and Neurodevelopment. Pharmaceuticals 2022, 15, 1533. [Google Scholar] [CrossRef]
- Lambert, A.M.; Bonkowsky, J.L.; Masino, M.A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. 2012, 32, 13488–13500. [Google Scholar] [CrossRef]
- Reimer, M.M.; Norris, A.; Ohnmacht, J.; Patani, R.; Zhong, Z.; Dias, T.B.; Kuscha, V.; Scott, A.L.; Chen, Y.-C.; Rozov, S.; et al. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev. Cell 2013, 25, 478–491. [Google Scholar] [CrossRef]
- Iannetta, A.; Caioni, G.; Di Vito, V.; Benedetti, E.; Perugini, M.; Merola, C. Developmental toxicity induced by triclosan exposure in zebrafish embryos. Birth Defects Res. 2022, 114, 175–183. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.; Hooyberghs, J.; Blust, R.; Witters, H.E. Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol. Teratol. 2013, 37, 44–56. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Patterson, J.; Kimmel, R.O. The development and behavioral characteristics of the startle response in the zebra fish. Dev. Psychobiol. 1974, 7, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Money, D.; Lee, T.; O’Brien, C.; Brophy, J.; Bitnun, A.; Kakkar, F.; Boucoiran, I.; Alimenti, A.; Vaudry, W.; Singer, J.; et al. Canadian Perinatal HIV Surveillance Program. Congenital anomalies following antenatal exposure to dolutegravir: A Canadian surveillance study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Lenis, M.G.; Laurette, E.Y.; Tejada, O.; Sanghvi, T.; Leung, K.-Y.; Cahill, L.S.; Sled, J.G.; Delgado-Olguín, P.; Greene, N.D.; et al. Dolutegravir in pregnant mice is associated with increased rates of fetal defects at therapeutic but not at supratherapeutic levels. EBioMedicine 2021, 63, 103167. [Google Scholar] [CrossRef] [PubMed]
- Tukeman, G.L.; Wei, H.; Finnell, R.H.; Cabrera, R.M. Dolutegravir induced neural tube defects in mice are folate responsive. AIDS 2024, 38, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Fought, A.J.; Sung, J.F.; McKinney, J.R.; Metz, T.D.; Fetters, K.B.; Lazarus, S.; Capraro, S.; Barr, E.; Glenny, C.; et al. Congenital malformations and preeclampsia associated with integrase inhibitor use in pregnancy: A single-center analysis. PLoS ONE 2023, 18, e0276473. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Watson, B.; Zhang, J.G.; Papp, E.; Lenis, M.G.; Dennehy, M.; Cameron, D.W.; Harrigan, P.R.; Serghides, L. Improving the clinical relevance of a mouse pregnancy model of antiretroviral toxicity; a pharmacokinetic dosing-optimization study of current HIV antiretroviral regimens. Antivir. Res. 2018, 159, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Beekhuijzen, M.; Beyer, B.; Chapin, R.; Dorau, M.; Hoberman, A.; Krupp, E.; Leconte, I.; Stedman, D.; Stethem, C.; et al. A multi-institutional study benchmarking the zebrafish developmental assay for prediction of embryotoxic plasma concentrations from rat embryo-fetal development studies. Reprod. Toxicol. 2019, 86, 33–44. [Google Scholar] [CrossRef]
- Gibbs, H.C.; Chang-Gonzalez, A.; Hwang, W.; Yeh, A.T.; Lekven, A.C. Midbrain-Hindbrain Boundary Morphogenesis: At the Intersection of Wnt and Fgf Signaling. Front. Neuroanat. 2017, 11, 64. [Google Scholar] [CrossRef]
- Scholpp, S.; Brand, M. Integrity of the midbrain region is required to maintain the diencephalic-mesencephalic boundary in zebrafish no isthmus/pax2.1 mutants. Dev. Dyn. 2003, 228, 313–322. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Woldstad, C.J.; Fang, M.; Bade, A.N.; McMillan, J.; Edagwa, B.; Boska, M.D.; Gendelman, H.E.; Siuzdak, G. Nanoformulated Antiretroviral Therapy Attenuates Brain Metabolic Oxidative Stress. Mol. Neurobiol. 2019, 56, 2896–2907. [Google Scholar] [CrossRef]
- Foster, E.G.; Sillman, B.; Liu, Y.; Summerlin, M.; Kumar, V.; Sajja, B.R.; Cassidy, A.R.; Edagwa, B.; Gendelman, H.E.; Bade, A.N. Long-acting dolutegravir formulations prevent neurodevelopmental impairments in a mouse model. Front. Pharmacol. 2023, 14, 1294579. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Mohan, H.; Ajaykumar, A.; Hsieh, A.Y.Y.; Martineau, L.; Patel, R.; Gadawska, I.; Sherwood, C.; Serghides, L.; Piret, J.M.; et al. Second-Generation Human Immunodeficiency Virus Integrase Inhibitors Induce Differentiation Dysregulation and Exert Toxic Effects in Human Embryonic Stem Cell and Mouse Models. J. Infect. Dis. 2022, 226, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.I.A.; Junger, A.L.; Caixeta, L.F.; Noll, M.; Oliveira, C.; Silveira, É.A. The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Kishida, R.; Yamagishi, K.; Ikeda, A.; Hayama-Terada, M.; Shimizu, Y.; Muraki, I.; Umesawa, M.; Imano, H.; Sankai, T.; Okada, T.; et al. Serum folate and risk of disabling dementia: A community-based nested case-control study. Nutr. Neurosci. 2024, 27, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.; Heisenberg, C.P. Zebrafish gastrulation: Putting fate in motion. Curr. Top. Dev. Biol. 2020, 136, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Leydier, L.; Sharma, S.; Katwala, J.; Sahu, A. A quest for genetic causes underlying signaling pathways associated with neural tube defects. Front. Pediatr. 2023, 11, 1126209. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Wang, J. Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? Int. J. Mol. Sci. 2023, 24, 2220. [Google Scholar] [CrossRef]
- Amacher, S.L.; Draper, B.W.; Summers, B.R.; Kimmel, C.B. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 2002, 129, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Marlow, F.; Gonzalez, E.M.; Yin, C.; Rojo, C.; Solnica-Krezel, L. No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 2004, 131, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Morley, R.H.; Lachani, K.; Keefe, D.; Gilchrist, M.J.; Flicek, P.; Smith, J.C.; Wardle, F.C. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc. Natl. Acad. Sci. USA 2009, 106, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.A.; Tümpel, S.; Dubrulle, J.; Schier, A.F.; Smith, J.C. No tail integrates two modes of mesoderm induction. Development 2010, 137, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Merker, S.; van Eeden, F.J.; Halpern, M.E.; Kimmel, C.B.; Nüsslein-Volhard, C. No tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 1994, 120, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, K.; Shimoda, N. De novo DNA methylation at the CpG island of the zebrafish no tail gene. Genesis 2003, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Lu, X.; Wang, S.; Wang, Z.; Huo, J.; Huang, J.; Shangguan, S.; Li, S.; Zou, J.; Bao, Y.; et al. The effect of folic acid deficiency on FGF pathway via Brachyury regulation in neural tube defects. FASEB J. 2019, 33, 4688–4702. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Bonner, J.R.; Bernard, D.J.; Sanchez, E.L.; Sause, E.T.; Prentice, R.R.; Burgess, S.M.; Brody, L.C. Disruption of the folate pathway in zebrafish causes developmental defects. BMC Dev. Biol. 2012, 12, 12. [Google Scholar] [CrossRef]
- Macdonald, R.; Scholes, J.; Strähle, U.; Brennan, C.; Holder, N.; Brand, M.; Wilson, S.W. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development 1997, 124, 2397–2408. [Google Scholar] [CrossRef]
- Blader, P.; Fischer, N.; Gradwohl, G.; Guillemot, F.; Strähle, U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development 1997, 124, 4557–4569. [Google Scholar] [CrossRef]
- Blader, P.; Plessy, C.; Strähle, U. Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech. Dev. 2003, 120, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Einhorn, Z.; Mercurio, S.; Lee, S.; Lau, B.; Mione, M.; Wilson, S.W.; Guo, S. Neurogenin1 is a determinant of zebrafish basal forebrain dopaminergic neurons and is regulated by the conserved zinc finger protein Tof/Fezl. Proc. Natl. Acad. Sci. USA 2006, 103, 5143–5148. [Google Scholar] [CrossRef] [PubMed]
- Korzh, V.; Sleptsova, I.; Liao, J.; He, J.; Gong, Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev. Dyn. 1998, 213, 92–104. [Google Scholar] [CrossRef]
- Mueller, T.; Wullimann, M.F. Expression domains of neuroD (nrd) in the early postembryonic zebrafish brain. Brain Res. Bull. 2002, 57, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Ronneberger, O.; Ryu, S.; Nitschke, R.; Driever, W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2011, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.; Kratochwil, C.F.; Ryu, S.; Schweitzer, J.; Driever, W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010, 518, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Roy, H. Folate deprivation induced neuroinflammation impairs cognition. Neurosci. Lett. 2023, 807, 137264. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef]
- Murray, L.K.; Jadavji, N.M. The role of one-carbon metabolism and homocysteine in Parkinson’s disease onset, pathology and mechanisms. Nutr. Res. Rev. 2019, 32, 218–230. [Google Scholar] [CrossRef]
- Duan, W.; Ladenheim, B.; Cutler, R.G.; Kruman, I.I.; Cadet, J.L.; Mattson, M.P. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002, 80, 101–110. [Google Scholar] [CrossRef]
- Lee, E.S.; Chen, H.; Soliman, K.F.; Charlton, C.G. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology 2005, 26, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Moens, A.L.; Kass, D.A. Tetrahydrobiopterin and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2439–2444. [Google Scholar] [CrossRef]
- Cornell, R.A.; Eisen, J.S. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development 2002, 129, 2639–2648, Erratum in Development 2002, 129, 3279. [Google Scholar] [CrossRef]
- Katz, H.R.; Menelaou, E.; Hale, M.E. Morphological and physiological properties of Rohon-Beard neurons along the zebrafish spinal cord. J. Comp. Neurol. 2021, 529, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.; Stokstad, E.L. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Levenson, C.W. Zinc regulation of transcriptional activity during retinoic acid-induced neuronal differentiation. J. Nutr. Biochem. 2013, 24, 1940–1944. [Google Scholar] [CrossRef]
- Sutherland, M.J.; Wang, S.; Quinn, M.E.; Haaning, A.; Ware, S.M. Zic3 is required in the migrating primitive streak for node morphogenesis and left-right patterning. Hum. Mol. Genet. 2013, 22, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Winata, C.L.; Kondrychyn, I.; Kumar, V.; Srinivasan, K.G.; Orlov, Y.; Ravishankar, A.; Prabhakar, S.; Stanton, L.W.; Korzh, V.; Mathavan, S. Genome wide analysis reveals Zic3 interaction with distal regulatory elements of stage specific developmental genes in zebrafish. PLoS Genet. 2013, 9, e1003852. [Google Scholar] [CrossRef]
- Jiang, Y.G.; Wang, Y.H.; Zhang, H.; Wang, Z.Y.; Liu, Y.Q. Effects of early-life zinc deficiency on learning and memory in offspring and the changes in DNA methylation patterns. Nutr. Neurosci. 2022, 25, 1001–1010. [Google Scholar] [CrossRef]
- Kirkwood-Johnson, L.; Katayama, N.; Marikawa, Y. Dolutegravir Impairs Stem Cell-Based 3D Morphogenesis Models in a Manner Dependent on Dose and Timing of Exposure: An Implication for Its Developmental Toxicity. Toxicol. Sci. 2021, 184, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Nagula, S.V.; Tardieu, G.G.; Saker, E.; Shoja, M.; Loukas, M.; Oskouian, R.J.; Tubbs, R.S. Update on the notochord including its embryology, molecular development, and patology: A primer for the clinician. Cureus 2017, 9, e1137. [Google Scholar] [CrossRef] [PubMed]
- Corallo, D.; Trapani, V.; Bonaldo, P. The notochord: Structure and functions. Cell. Mol. Life Sci. 2015, 72, 2989–3008. [Google Scholar] [CrossRef] [PubMed]
- Zizioli, D.; Ferretti, S.; Mignani, L.; Castelli, F.; Tiecco, G.; Zanella, I.; Quiros-Roldan, E. Developmental safety of nirmatrelvir in zebrafish (Danio rerio) embryos. Birth Defects Res. 2023, 115, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/salts/DBSALT000943 (accessed on 13 February 2024).
- Christou, M.; Kavaliauskis, A.; Ropstad, E.; Fraser, T.W.K. DMSO effects larval zebrafish (Danio rerio) behavior, with additive and interaction effects when combined with positive controls. Sci. Total Environ. 2020, 709, 134490. [Google Scholar] [CrossRef] [PubMed]
- Hoyberghs, J.; Bars, C.; Ayuso, M.; Van Ginneken, C.; Foubert, K.; Van Cruchten, S. DMSO Concentrations up to 1% are Safe to be Used in the Zebrafish Embryo Developmental Toxicity Assay. Front. Toxicol. 2021, 3, 804033. [Google Scholar] [CrossRef]
- Zizioli, D.; Zanella, I.; Mignani, L.; Degli Antoni, M.; Castelli, F.; Quiros-Roldan, E. Cabotegravir Exposure of Zebrafish (Danio rerio) Embryos Impacts on Neurodevelopment and Behavior. Int. J. Mol. Sci. 2023, 24, 1994. [Google Scholar] [CrossRef]
- von Hellfeld, R.; Brotzmann, K.; Baumann, L.; Strecker, R.; Braunbeck, T. Adverse effects in the fish embryo acute toxicity (FET) test: A catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ. Sci. Eur. 2020, 32, 122. [Google Scholar] [CrossRef]
- OECDiLibrary. 2013. Available online: https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en (accessed on 13 February 2024).
- Zizioli, D.; Ferretti, S.; Tiecco, G.; Mignani, L.; Monti, E.; Castelli, F.; Quiros-Roldan, E.; Zanella, I. Comparison of Efavirenz and Doravirine Developmental Toxicity in an Embryo Animal Model. Int. J. Mol. Sci. 2023, 24, 11664. [Google Scholar] [CrossRef]

| Morphological Endpoints | Normal | Mild | Severe |
|---|---|---|---|
| Head | Flexed | Straight | Extended |
| Tail | Properly detached and straight | Slightly shorter length and curved | Shorter length and curved |
| Anterior posterior (A-P) axis | Normal head and tail position | AP axis mildly disrupted | AP axis not well formed |
| Somites | V-shaped | U-shaped | Straight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizioli, D.; Quiros-Roldan, E.; Ferretti, S.; Mignani, L.; Tiecco, G.; Monti, E.; Castelli, F.; Zanella, I. Dolutegravir and Folic Acid Interaction during Neural System Development in Zebrafish Embryos. Int. J. Mol. Sci. 2024, 25, 4640. https://doi.org/10.3390/ijms25094640
Zizioli D, Quiros-Roldan E, Ferretti S, Mignani L, Tiecco G, Monti E, Castelli F, Zanella I. Dolutegravir and Folic Acid Interaction during Neural System Development in Zebrafish Embryos. International Journal of Molecular Sciences. 2024; 25(9):4640. https://doi.org/10.3390/ijms25094640
Chicago/Turabian StyleZizioli, Daniela, Eugenia Quiros-Roldan, Sara Ferretti, Luca Mignani, Giorgio Tiecco, Eugenio Monti, Francesco Castelli, and Isabella Zanella. 2024. "Dolutegravir and Folic Acid Interaction during Neural System Development in Zebrafish Embryos" International Journal of Molecular Sciences 25, no. 9: 4640. https://doi.org/10.3390/ijms25094640





