The Impact of Thallium Exposure in Public Health and Molecular Toxicology: A Comprehensive Review
Abstract
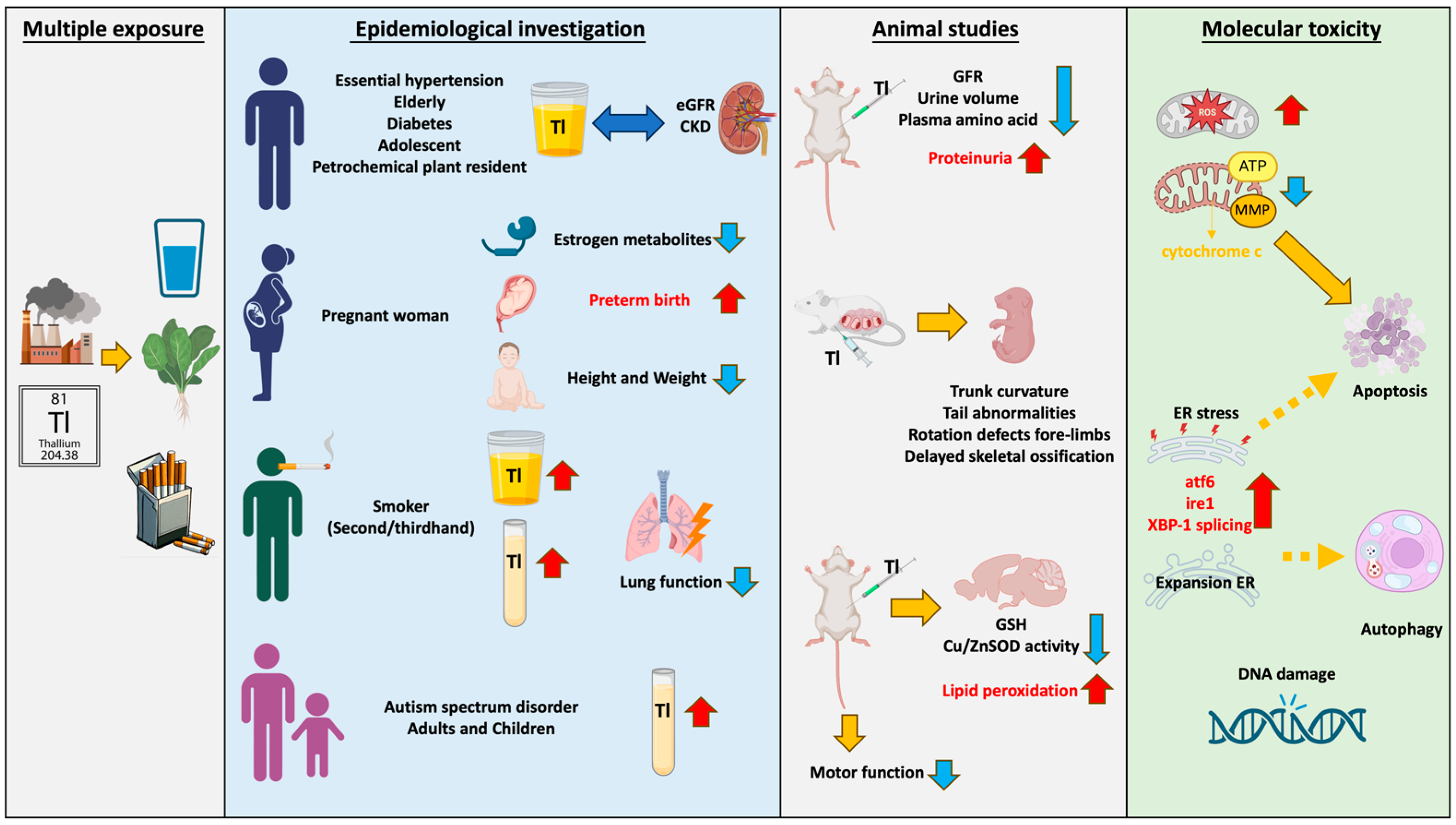
:1. Introduction
2. Thallium Exposure in Daily Life
2.1. Thallium in Beverages
2.2. Thallium in Tobaccos
2.3. Thallium in Vegetables
3. Epidemiological Investigation of Thallium
3.1. The Effects of Thallium on Human Kidney Health
3.2. The Effects of Thallium on the Health of Children and Pregnant Women
3.3. The Effects of Thallium on the Health of Smokers
3.4. Thallium Causes Psychological, Metabolic, and Other Effects
4. Molecular Toxicity and Adverse Reactions Caused by Thallium
4.1. Mitochondria-Mediated Oxidative Stress
4.2. Thallium Induces Cellular Toxicity by Eliciting Endoplasmic Reticulum Stress
4.3. Genotoxicity and other Adverse Effects of Thallium
4.4. The Toxicity and Adverse Reactions of Thallium in Mammalian Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Gu, S. Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance. Minerals 2021, 11, 855. [Google Scholar] [CrossRef]
- Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Qi, J.; Liu, J.; Wang, J.; Lin, K.; Ouyang, Q.; Zhang, X.; Wei, X.; Xiao, T.; El-Naggar, A.; et al. Thallium isotopic compositions as tracers in environmental studies: A review. Environ. Int. 2022, 162, 107148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Dong, X.; Yin, M.; Tsang, D.C.W.; Sun, J.; Liu, J.; Song, G.; Liu, Y. Temporal sedimentary record of thallium pollution in an urban lake: An emerging thallium pollution source from copper metallurgy. Chemosphere 2020, 242, 125172. [Google Scholar] [CrossRef] [PubMed]
- Vaněk, A.; Chrastný, V.; Komárek, M.; Penížek, V.; Teper, L.; Cabala, J.; Drábek, O. Geochemical position of thallium in soils from a smelter-impacted area. J. Geochem. Explor. 2013, 124, 176–182. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed]
- Campanella, B.; Onor, M.; D’Ulivo, A.; Giannecchini, R.; D’Orazio, M.; Petrini, R.; Bramanti, E. Human exposure to thallium through tap water: A study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy). Sci. Total Environ. 2016, 548–549, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; D’Orazio, M.; Doveri, M.; Lelli, M.; Petrini, R.; Giannecchini, R. Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J. Geochem. Explor. 2019, 197, 84–92. [Google Scholar] [CrossRef]
- Li, F.; Jing, M.; Ma, F.; Wang, W.; Li, M. Comparison and Risk Assessment of Macroelements and Trace Metals in Commercial Teas from Different Regions of China. Biol. Trace Elem. Res. 2023, 201, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, G.; Liu, K.; Yu, R.; Lu, Q.; Zhang, Y. Potential exposure to metals and health risks of metal intake from Tieguanyin tea production in Anxi, China. Environ. Geochem. Health 2019, 41, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Albals, D.; Al-Momani, I.F.; Issa, R.; Yehya, A. Multi-element determination of essential and toxic metals in green and roasted coffee beans: A comparative study among different origins using ICP-MS. Sci. Prog. 2021, 104, 368504211026162. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L’Abbate, L.; Longo, V.; Colombo, P.; Frasca, D.; Balzan, M.; Cuttitta, G.; et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM(2.5). Environ. Res. 2018, 165, 71–80. [Google Scholar] [CrossRef]
- Karbowska, B.; Zembrzuski, W. Determining Thallium in a Commercial Tobacco Brand Available in Poland. Pol. J. Environ. Stud. 2016, 25, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Polzin, G.M.; Zhang, L.; Watson, C.H.; Paschal, D.C.; Ashley, D.L. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem. Toxicol. 2006, 44, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Polzin, G.M.; Watson, C.H.; Ashley, D.L. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic US brands. Food Chem. Toxicol. 2007, 45, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Pavlickova, J.; Zbiral, J.; Smatanova, M.; Habarta, P.; Houserova, P.; Kuban, V. Uptake of thallium from naturally-contaminated soils into vegetables. Food Addit. Contam. 2006, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, X.; Wang, J.; Xiao, T.; Chen, D.; Sheng, G.; Yin, M.; Lippold, H.; Wang, C.; Chen, Y. Thallium contamination in arable soils and vegetables around a steel plant-A newly-found significant source of Tl pollution in South China. Environ. Pollut. 2017, 224, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, N.; Zhang, W.; Wei, X.; Tsang, D.C.W.; Sun, Y.; Luo, X.; Bao, Z.; Zheng, W.; Wang, J.; et al. Thallium contamination in farmlands and common vegetables in a pyrite mining city and potential health risks. Environ. Pollut. 2019, 248, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef] [PubMed]
- Umweltqualität, F. Maximum Emission Values–Maximum Thallium Emission Values for Livestock (Richtlinie 2310 Blatt 29 (E)); Kommission Reinhaltung der Luft im VDI und DIN–Normenausschuss KRdL: Düsseldorf, Germany, 1998. [Google Scholar]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, R.; He, P.; Zhang, Z.; Dai, Y.; Li, M.; Liu, Z.; Yang, H.; Guan, S.; Sun, J. Association between urinary metal concentrations and abnormal estimated glomerular filtration rate in Chinese community-dwelling elderly: Exploring the mediating effect of triglycerides. Ecotoxicol. Environ. Saf. 2023, 259, 114966. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018, 112, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Hu, B.; Meng, X.L.; Sun, L.; Li, H.B.; Xu, P.R.; Cheng, B.J.; Sheng, J.; Tao, F.B.; Yang, L.S.; et al. The associations between urinary metals and metal mixtures and kidney function in Chinese community-dwelling older adults with diabetes mellitus. Ecotoxicol. Environ. Saf. 2021, 226, 112829. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Vargas, G.G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.J.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014, 132, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Ke, D.Y.; Wang, J.E.; Chan, C.C. Associations between renal functions and exposure of arsenic and polycyclic aromatic hydrocarbon in adults living near a petrochemical complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ Int 2018, 121, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.; Malmqvist, E.; Saborit, A.; Gratacos, E.; Gomez Roig, M.D. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS ONE 2017, 12, e0185645. [Google Scholar] [CrossRef] [PubMed]
- Ziskoven, R.; Achenbach, C.; Schulten, H.R.; Roll, R. Thallium determinations in fetal tissues and maternal brain and kidney. Toxicol. Lett. 1983, 19, 225–231. [Google Scholar] [CrossRef]
- Li, X.; Li, A.; Zhang, W.; Liu, X.; Liang, Y.; Yao, X.; Song, M. A pilot study of mothers and infants reveals fetal sex differences in the placental transfer efficiency of heavy metals. Ecotoxicol. Environ. Saf. 2019, 186, 109755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, W.; Zhang, B.; Pan, X.; Liu, W.; Jin, S.; Huo, W.; Liu, H.; Peng, Y.; Sun, X.; et al. Predictors of thallium exposure and its relation with preterm birth. Environ. Pollut. 2018, 233, 971–976. [Google Scholar] [CrossRef]
- Qi, J.; Lai, Y.; Liang, C.; Yan, S.; Huang, K.; Pan, W.; Feng, L.; Jiang, L.; Zhu, P.; Hao, J.; et al. Prenatal thallium exposure and poor growth in early childhood: A prospective birth cohort study. Environ. Int. 2019, 123, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, Y.; Song, L.; Liu, B.; Zhang, L.; Wang, L.; Liu, Y.; Bi, J.; Xiong, C.; Cao, Z.; et al. Prenatal exposure to thallium is associated with decreased mitochondrial DNA copy number in newborns: Evidence from a birth cohort study. Environ. Int. 2019, 129, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, L.; Song, L.; Liu, B.; Liu, Y.; Bi, J.; Liu, Q.; Chen, K.; Li, Y.; Xia, W.; et al. The association between prenatal exposure to thallium and shortened telomere length of newborns. Chemosphere 2021, 265, 129025. [Google Scholar] [CrossRef] [PubMed]
- Maitre, L.; Robinson, O.; Martinez, D.; Toledano, M.B.; Ibarluzea, J.; Marina, L.S.; Sunyer, J.; Villanueva, C.M.; Keun, H.C.; Vrijheid, M.; et al. Urine Metabolic Signatures of Multiple Environmental Pollutants in Pregnant Women: An Exposome Approach. Environ. Sci. Technol. 2018, 52, 13469–13480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liang, C.; Yan, S.; Li, Z.; Huang, K.; Xia, X.; Hao, J.; Zhu, P.; Tao, F. Association between serum thallium in early pregnancy and risk of gestational diabetes mellitus: The Ma’anshan birth cohort study. J. Trace Elem. Med. Biol. 2019, 52, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Molavi, N.; Ghaderi, A.; Banafshe, H.R. Determination of thallium in urine, blood, and hair in illicit opioid users in Iran. Hum. Exp. Toxicol. 2020, 39, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; NasehGhafoori, P.; Rasouli-Azad, M.; Sehat, M.; Mehrzad, F.; Nekuei, M.; Aaseth, J.; Banafshe, H.R.; Mehrpour, O. Examining of Thallium in Cigarette Smokers. Biol. Trace Elem. Res. 2018, 182, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Amirabadizadeh, A.; Ataei, M.; Ataei, H.; Zardast, M.; Shariatmadari, M.R.; Mousavi-Mirzaei, S.M.; Mehrpour, O. Comparison of serum concentrations of essential and toxic elements between cigarette smokers and non-smokers. Environ. Sci. Pollut. Res. Int. 2021, 28, 37672–37678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Li, Z.C.; Zhou, Y.; Dong, C.Y.; Kuang, H.X.; Zheng, T.; Xiang, M.D.; Chen, X.C.; Li, H.Y.; Zeng, X.W.; et al. Associations between trace level thallium and multiple health effects in rural areas: Chinese Exposure and Response Mapping Program (CERMP). Sci. Total Environ. 2023, 862, 160466. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Bai, Y.; Feng, W.; Wang, S.; Chen, Z.; Fu, W.; Li, G.; Chen, W.; Wang, G.; et al. Effect of thallium exposure and its interaction with smoking on lung function decline: A prospective cohort study. Environ. Int. 2019, 127, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Li, Y.; Liu, Y.; Zhao, Z. Blood Mercury, Arsenic, Cadmium, and Lead in Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2018, 181, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, D.; Xu, C.; Wang, J.; Jin, Y. Hair levels of heavy metals and essential elements in Chinese children with autism spectrum disorder. J. Trace Elem. Med. Biol. 2021, 66, 126748. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Howsmon, D.P.; Kruger, U.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.; Mitchell, J.; Ingram, J.; Hellmers, R.; et al. Significant Association of Urinary Toxic Metals and Autism-Related Symptoms-A Nonlinear Statistical Analysis with Cross Validation. PLoS ONE 2017, 12, e0169526. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef] [PubMed]
- Yorita Christensen, K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007-2008. Int J Hyg Environ Health 2013, 216, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public. Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Nishimoto, N. Thallium–poisoner’s poison: An overview and review of current knowledge on the toxicological effects and mechanisms. Curr. Res. Toxicol. 2024, 6, 100157. [Google Scholar] [CrossRef] [PubMed]
- Rusznyák, I.; György, L.; Ormai, S.; Millner, T. On some potassium-like qualities of the thallium ion. Experientia 1968, 24, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Shaheen, S.M.; El-Naggar, A.; Wang, H.; Du Laing, G.; Alessi, D.S.; Sik Ok, Y. Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ. Int. 2020, 140, 105754. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Tan, S.W.; Cheng, C.J.; Chen, P.J. Revealing the toxicity of monovalent and trivalent thallium to medaka fish in controlled exposure conditions. Aquat. Toxicol. 2022, 250, 106258. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium toxicity in humans. Arh. Hig. Rada Toksikol. 2010, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Arzate, S.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Maldonado, P.D.; Vazquez-Roman, B.; Rios, C.; Santamaria, A. Delayed effects of thallium in the rat brain: Regional changes in lipid peroxidation and behavioral markers, but moderate alterations in antioxidants, after a single administration. Food Chem. Toxicol. 2005, 43, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium Use, Toxicity, and Detoxification Therapy: An Overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, J.F.; Chen, P.J.; Huang, Y.T.; Liu, B.H. Thallium exposure interfered with heart development in embryonic zebrafish (Danio rerio): From phenotype to genotype. Sci. Total Environ. 2023, 878, 162901. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.R.; Mashayekhi, V.; Aslani, M.; Hosseini, M.J. Toxicity of thallium on isolated rat liver mitochondria: The role of oxidative stress and MPT pore opening. Environ. Toxicol. 2015, 30, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Eskandari, M.R.; Daraei, B. A comparison of hepatocyte cytotoxic mechanisms for thallium (I) and thallium (III). Environ. Toxicol. 2010, 25, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.R.; Pourahmad, J.; Daraei, B. Thallium(I) and thallium(III) induce apoptosis in isolated rat hepatocytes by alterations in mitochondrial function and generation of ROS. Toxicol. Environ. Chem. 2010, 93, 145–156. [Google Scholar] [CrossRef]
- Hanzel, C.E.; Verstraeten, S.V. Thallium induces hydrogen peroxide generation by impairing mitochondrial function. Toxicol. Appl. Pharmacol. 2006, 216, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.T.L.; Marotte, C.; Verstraeten, S.V. Epidermal growth factor prevents thallium(I)- and thallium(III)-mediated rat pheochromocytoma (PC12) cell apoptosis. Arch. Toxicol. 2017, 91, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity Induced by Low Thallium Doses in Living Hippocampal Neurons: Evidence of Early Onset Mitochondrial Dysfunction and Correlation with Ethanol Production. ACS Chem. Neurosci. 2019, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Sun, Y.; Long, J.; Sui, X.; Yang, J.; Wang, Q.; Wang, S.; He, H.; Luo, Y.; Qiu, Z.; et al. Involvement of the Nrf2-Keap1 signaling pathway in protection against thallium-induced oxidative stress and mitochondrial dysfunction in primary hippocampal neurons. Toxicol. Lett. 2020, 319, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef] [PubMed]
- Morel Gomez, E.; Casali, C.I.; Fernandez, M.D.C.; Verstraeten, S.V. Tl(I) and Tl(III) induce reticulum stress in MDCK cells. Environ. Toxicol. Pharmacol. 2023, 101, 104192. [Google Scholar] [CrossRef] [PubMed]
- Ozgul Ozalp, F.; Kutlu, M.; Iscan, A. The Effects of Thallium Acetate on Hepatopancreatic Cells of Gammarus pulex (Crustacea: Amphipoda). Ekoloji 2011, 20, 15–20. [Google Scholar] [CrossRef]
- Rodríguez-Mercado, J.J.; Mosqueda-Tapia, G.; Altamirano-Lozano, M.A. Genotoxicity assessment of human peripheral Lymphocytes induced by thallium(I) and thallium(III). Toxicol. Environ. Chem. 2017, 99, 987–998. [Google Scholar] [CrossRef]
- Rodriguez-Mercado, J.J.; Hernandez-de la Cruz, H.; Felipe-Reyes, M.; Jaramillo-Cruz, E.; Altamirano-Lozano, M.A. Evaluation of cytogenetic and DNA damage caused by thallium(I) acetate in human blood cells. Environ. Toxicol. 2015, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Alonzo, R.; Núñez-Tapia, F.A.; Moreno-Godínez, M.E.; Talavera-Mendoza, O.; Ortuño-Pineda, C.; Quintanilla-Vega, B.; Solís-Heredia, M.J.; Rodríguez-Mercado, J.J.; Urióstegui-Acosta, M. Effect of quality sperm and DNA damage by thallium exposure in mice mature sperm. Toxicol. Lett. 2016, 259, S232–S233. [Google Scholar] [CrossRef]
- Repetto, G.; Sanz, P.; Repetto, M. In vitro effects of thallium on mouse neuroblastoma cells. Toxicol. Vitr. 1994, 8, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, L.; Yao, H.; Su, Q.; Ye, J. Thallium exposure induces changes in B and T cell generation in mice. Toxicology 2023, 492, 153532. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Appenroth, D. Renal amino acid transport in immature and adult rats during thallium-induced nephrotoxicity. Toxicology 1996, 106, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, D.; Gambaryan, S.; Winnefeld, K.; Leiterer, M.; Bräunlich, H. Functional and morphological aspects of thallium-induced nephrotoxicity in rats. Toxicology 1995, 96, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arzate, S.; Martínez, A.; Medina, E.; Santamaría, A.; Ríos, C. Subchronic administration of sublethal doses of thallium to rats: Effects on distribution and lipid peroxidation in brain regions. Toxicol. Lett. 2000, 116, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Barrera, L.; Rodriguez-Mercado, J.J.; Mateos-Nava, R.A.; Vazquez-Martinez, Y.; Altamirano-Lozano, M.A. Effect on the offspring of pregnant females CD-1 mice treated with a single thallium(I) application. Reprod. Toxicol. 2019, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Sample | Thallium Concentrations | Area | Ref. |
|---|---|---|---|
| Tap water (spring water) | 10 (ppb) | Tuscany, Italy | [7] |
| Tap water (spring water) | 27.8 (ppb) | Northwestern Tuscany, Italy | [8] |
| Tea leaves | 4–409 (ppm) | Southwest, South, Jiangnan and Jiangbei tea regions of China | [9] |
| Soak tea | 3.86 × 10−4 (ppm) | Anxi, China | [10] |
| Roasted coffee beans | 2 ± 1 (ppb) | Brazil | [11] |
| Thallium Concentrations | Area | Summary | Ref. |
|---|---|---|---|
| 0.0089 ± 0.0012 (μg/g) | Poland | [13] | |
| 1.1 ± 0.1 to 2.4 ± 0.2 (ng/cigarette) | USA | [14] | |
| 0.70 ± 0.04 to 7.04 ± 0.23 (ng/cigarette) | USA | Mean smoke particulate thallium levels from counterfeit cigarettes were 1.4–4.9 times higher than authentic cigarettes. | [15] |
| Vegetable | Tissue | Thallium Concentrations (mg/kg, Dry Weight) | Area | Ref. |
|---|---|---|---|---|
| Snow peas | In edible parts | 1.47 ± 0.04 | Guangdong Province, China | [18] |
| Napa cabbage | In edible parts | 0.49 ± 0.01 | Guangdong Province, China | [18] |
| Chard | In edible parts | 1.65 ± 0.05 | Guangdong Province, China | [18] |
| Scallions | In edible parts | 0.77 ± 0.02 | Guangdong Province, China | [18] |
| Taiwanese lettuce | In edible parts | 0.92 ± 0.03 | Guangdong Province, China | [18] |
| Pak choi | In edible parts | 1.45 ± 0.04 | Guangdong Province, China | [18] |
| Crown daisy | In edible parts | 6.29 ± 0.26 | Yunfu City, China | [19] |
| Baby bok choy | In edible parts | 0.84 ± 0.03 | Yunfu City, China | [19] |
| Romaine lettuce | In edible parts | 0.49 ± 0.02 | Yunfu City, China | [19] |
| Watercress | In edible parts | 15.4 ± 0.51 | Yunfu City, China | [19] |
| Gai lan | In edible parts | 0.63 ± 0.02 | Yunfu City, China | [19] |
| Oilseed rape | In edible parts | 1.28 ± 0.05 | Yunfu City, China | [19] |
| Sweet potato | In edible parts | 2.78 ± 0.12 | Yunfu City, China | [19] |
| Chinese mustard | In edible parts | 0.56 ± 0.02 | Yunfu City, China. | [19] |
| Bok choy | In edible parts | 1.23 ± 0.05 | Yunfu City, China | [19] |
| Green cabbage | In edible parts | 0.85 ± 0.04 | Yunfu City, China | [19] |
| Sonchus lingiaus | stems and leaves | 0.72 ± 0.03 | Pearl River, China | [20] |
| Allium fistulosum L | stems and leaves | 0.92 ± 0.04 | Pearl River, China | [20] |
| Subject Description | Subject Number | Specimen Type | Area | Summary of Effect | Ref. |
|---|---|---|---|---|---|
| Aged 65 years or older | 37,523 | Urine | Yinchuan, China | Tl was negatively correlated with abnormal eGFR. | [23] |
| Aged above 30 years old, essential hypertension | 934 | Urine | Wuhan, China | Tl was positively associated with eGFR in male participants. | [24] |
| Aged above 60 years, diabetic | 592 | Urine | Fuyang City, Anhui, China | Negative association between Tl concentrations and CKD | [25] |
| Adolescent 11–16 years old | 512 | Urine | Torreón, Mexico | Tl is positively correlated with eGFR. | [26] |
| Least 35 years old, living near a petrochemical complex | 2069 | Urine | Yunlin County, Taiwan | Decreased eGFR, higher odds of having CKD. | [27] |
| Subject Description | Subject Number | Specimen Type | Area | Summary of Effect | Ref. |
|---|---|---|---|---|---|
| Mother–infant pairs | 7173 | Urine | Wuhan, China | Maternal exposure to Tl is related to increased risk of preterm birth. | [32] |
| Mother–infant pairs (Ages 0–2 year, fetus) | 3080 | Maternal serum/umbilical cord serum | Anhui, China | Umbilical cord serum Tl levels were associated with reduced height and weight in young girls. | [33] |
| Mother–newborn pairs | 746 | Uurine/umbilical cord blood | Wuhan, China | Maternal urinary Tl is associated with reduced mtDNAcn leukocytes in cord blood. | [34] |
| Mother–infant pairs | 746 | Urine/umbilical cord serum | Wuhan, China | Maternal exposure to thallium during delivery is associated with shortened telomere length in newborns. | [35] |
| Pregnant woman | 750 | Urine/blood/umbilical cord serum | Spain | Urinary thallium is positively correlated with scyllo-inositol, acetate, formate, dimethylamine. Urinary thallium is negatively correlated with N-acetyl neuraminic acid and trans-aconitate. Positive associations with pregnanolone-3-glucuronide were particularly strong in the first trimester and negative associations with estrogen metabolites in the third trimester. | [36] |
| Pregnant woman | 3013 | Blood | Anhui, China | Serum thallium concentration significantly associated with risk of gestational diabetes mellitus (advanced age). | [37] |
| Subject Description | Smokers | Non- Smokers | Specimen Type | Area | Summary of Effect | Ref. |
|---|---|---|---|---|---|---|
| Age range of 23 to 77 years | 56 | 53 | Urine | Iran | The mean value (with SD) for urinary thallium in the smokers (10.16 ± 1.82 μg/L) was significantly higher than in the control group (2.39 ± 0.63 μg/L). | [39] |
| Age range of 19 to 74 years | 100 | 100 | Serum | USA | This study indicated that the level of thallium was higher in smokers than in non-smokers. Tl (0.54 [0.27–0.68] versus 0.34 [0.11–0.66] (μg/L), p = 0.04). | [40] |
| Age range of 21 to 81 years | 50 | 50 | Urine/blood/hair | Iran | There were significant correlations between duration of illicit opioid use and urine thallium concentrations (r = 0.394, p = 0.005) and hair thallium concentrations (r = 0.293, p = 0.039). | [38] |
| Age range of 10 to 15 years | 27 | 46 | Indoor/outdoor sampling of PM 2.5 | Italy | Indoor smoking is associated with elevated Tl levels and is associated with increased respiratory symptoms in children. | [16] |
| Aged above 18 years | 526 | 1837 | Urine/serum | Central, east, northeast, north, northwest, south, and southwest China | Tl was negatively associated with increases in lung health indicators. | [41] |
| Workers from a coke oven plant | 790 | 453 | Urine | Wuhan, China | Urinary thallium is associated with increased lung function decline, especially in smokers. | [42] |
| Subject Description | Subject Number | Specimen Type | Area | Summary of Effect | Ref. |
|---|---|---|---|---|---|
| Age 5–16 years old | 44 | Urine | Arizona, USA | Children with autism spectrum disorder (ASD), on average, had higher levels of thallium. | [46] |
| Age of 2.5 years to 60 years | 67 | Urine | Arizona, USA | Urinary thallium is positively correlated with autism spectrum disorder. | [47] |
| 65.53 ± 6.37 years | 118 | Serum | Italy | Serum thallium correlates with subjective memory complaints and Alzheimer’s disease. | [48] |
| Aged above 20 years | 1587 | Urine | USA | Urinary thallium is associated with decreased Thyroxine (T4). | [49] |
| Aged above 18 years | 2363 | Urine/serum | Central, east, northeast, north, northwest, south, and southwest China | Tl > 0.40 μg/g was positively associated with increases in serum bilirubin. | [41] |
| 6–60 years old | 3816 | Urine | USA | Thallium is positively associated with body mass index (BMI) and waist circumference (WC). | [50] |
| Aged 20 to 26 years | 53 | Urine | Guangzhou, China | The levels of Tl correlated significantly with the urinary 8-hydroxy-2-deoxyguanosine (8-OHdG) level. | [51] |
| Experimental Animals/Materials | Thallium Compounds | Concentrations | Summary of Effect | Ref. |
|---|---|---|---|---|
| Isolated rat liver (male Sprague Dawley) | Thallium (I) nitrate | 25–200 μM | Increase in mitochondrial ROS formation, ATP consumption, GSH oxidation, mitochondrial outer membrane rupture, mitochondrial swelling, MMP collapse, and cytochrome c release. Effect dose: 25 μM. | [60] |
| Hepatocyte Male Sprague Dawley | Thallium (I) nitrate Thallium (III) nitrate | Tl (I) 200 μM Tl (III) 50 μM | Generation of reactive oxygen species (ROS), mitochondrial membrane potential collapse, activation of the caspase cascade, and the appearance of the cellular apoptosis phenotype. Effect dose: Tl (I) 200 and Tl (III) 50 μM. | [61] |
| Hepatocyte Male Sprague Dawley | Thallium (I) nitrate Thallium (III) nitrate | Tl (I) 200 μM Tl (III) 50 μM | Inducing ROS generation, lipid peroxidation, mitochondrial membrane potential collapse, activation of the caspase cascade, and lysosomal membrane leakage. Tl (I) 200 and Tl (III) 50 μM. | [62] |
| Rat adrenal pheochromocytoma (PC12 cells) | Thallium (I) nitrate Thallium (III) nitrate | 10–250 μM | Tl(I) and Tl (III) significantly increase mitochondrial H2O2 steady-state levels. The glutathione content is significantly reduced in cells treated with Tl. There is a higher level of oxidants in the cytoplasm, which is positively correlated with the mitochondrial H2O2 content. Effect dose: 10 μM. | [63] |
| Rat pheochromocytoma (PC12) | Thallium(I) nitrate Thallium (III) nitrate | 25–100 μM | Reduce mitochondrial membrane potential, enhance H2O2 generation, and activate mitochondria-dependent cell apoptosis. Tl (III) increases nitric oxide production, leading to an imbalance between anti-apoptotic and pro-apoptotic members of the Bcl-2 family. Effect dose: 25 μM. | [64] |
| Murine hippocampal neuroblasts (HN9.10e) | Thallium (I) chloride | 1–100 μg/L | Neurite shortening, loss of substrate adhesion, and an increase in cytoplasmic calcium. Dose-dependent changes in mitochondrial ROS (mtROS) levels and transmembrane mitochondrial membrane potential (ΔΨm). Effect dose: 10 μg/L. | [65] |
| Primary hippocampal neuron E17-E18 Wistar rat embryos | Thallium (I) nitrate | 100–200 μM | The Nrf2-Keap1 pathway can prevent thallium-induced oxidative stress and mitochondrial dysfunction. Effect dose: 100 μM. | [66] |
| Experimental Animals/materials | Thallium Compounds | Concentrations | Summary of Effect | Ref. |
|---|---|---|---|---|
| Madin–Darby Canine Kidney cells | Thallium (I) nitrate Thallium (III) nitrate | Tl (I) 10,100 μM Tl (III) 10,100 μM | The expression of endoplasmic reticulum (ER) stress markers ATF-6 and IRE-1 increase by 100% and 150%, respectively, accompanied by XBP-1 splicing and nuclear translocation. Effect dose: 10 μM. | [68] |
| Gammarus pulex | Thallium (I) acetate | 0.1–0.6 mg/L | Observations through transmission electron microscopy revealed the fragmentation and expansion of the rough endoplasmic reticulum (RER). Additionally, an increase in the quantity of lipid droplets, lysosomes, and autophagic vesicles was observed within liver cells. Effect dose: 0.2 mg/L. | [69] |
| Experimental Animals/Materials | Thallium Compounds | Subject Number | Concentrations | Summary of Effect | Ref. |
|---|---|---|---|---|---|
| Human lymphocyte | Thallium (I) sulfate Thallium (III) chloride | 0.5, 1, 5, 50, 100 μg/mL | Treatment with Tl (I) and Tl (III) significantly increases structural chromosomal abnormalities with or without gaps and raises the percentage of cells with abnormalities without gaps. Effect dose: 0.5 mg/L. | [70] | |
| Human blood cells | Thallium (I) acetate | 0.5, 1, 5, 10, 50, 100 µg/mL | Increased comet assay length. Mitotic and replication indexes exhibit a significant dose-dependent decrease. Effect dose: 0.5 mg/L. | [71] | |
| Male mouse | Thallium (I) | Not disclosed | Adult male mice were administered Tl (15 or 25 mg/kg bw, orally, once a day for 5 days), and euthanized 24 h post-treatment. | Both sperm viability and motility decrease. DFI% (fragmented DNA) increases. Effect dose: 15 mg/kg bw. | [72] |
| Mouse neuroblastoma cells (Neuro-2A) | Thallium (I) acetate | 0, 0.1, 1, 10, 100, 1000 mg/L | The activity of acetylcholinesterase is highly sensitive to Tl inhibition. Effect dose: 100 mg/L. | [73] | |
| C57BL/6J male mice (specific-pathogen-free, SPF) | Thallium (I) nitrate | 15 | 50 mg/L(drink). Euthanasia was performed after continuous exposure for one week. | Tl (I) increases apoptosis in bone marrow, suppresses the expression of genes involved in B cell development, enhances the production of thymic CD4+ T cells, and promotes the migration of initial CD4+ T cells and recent thymic emigrants (RTE) from the thymus to the spleen. Effect dose: 50 mg/L. | [74] |
| Experimental Animals/Materials | Thallium Compounds | Subject Number | Concentrations | Summary of Effect | Ref. |
|---|---|---|---|---|---|
| Wistar rats (female) | Thallium (I) sulfate | 6 | 5, 10, 15, 20 mg Tl2SO4/kg body weight, intraperitoneally. A single intraperitoneal injection is administered on either day 10 or day 55 after birth. | The glomerular filtration rate (GFR) and urine output decrease, while proteinuria increases. Effect dose: Tl, 2 mg/100 g b.wt. | [75] |
| Wistar rats (female) | Thallium (I) sulfate | 6 | 5, 10, 15, 20 mg Tl2SO4/kg body weight, intraperitoneally. After a single dose of T1 administration, observations were made for up to 10 days. | The glomerular filtration rate (GFR) decreased and urine output reduced, while proteinuria increased. Effect dose: Tl, 20 mg/kg b.wt. | [76] |
| Male Wistar rats | Thallium (I) acetate | 6 | 8 or 16 mg/kg i.p. At 1 day, 3 days, and 7 days post T1+ administration, rats were euthanized by decapitation, and their brains were rapidly removed. | After administration of Tl for 7 days, there is a dose-dependent accumulation observed in the brain region. Lipid peroxidation increases in the hypothalamus (Ht), cerebellum (Ce), and striatum (S), while the activity of Cu,Zn-superoxide dismutase (SOD) decreases in both Ht and S. Additionally, animals exhibit a general decline in motor function. Effect dose: 8 mg/kg. | [57] |
| Adult male bred-in-house Wistar rats | Thallium (I) acetate | 6–10 | Rats were administered T1 acetate solution intraperitoneally daily for 30 days at doses of 0.8 mg/kg or 1.6 mg/kg. On the third day after the treatment concluded, the animals were euthanized by decapitation. | Significant changes in lipid peroxidation occur in the rat’s brain. Effect dose: 0.8 mg/kg. | [77] |
| Sexually mature CD-1 mice | Thallium (I) acetate | 10 | A single intraperitoneal injection was administered to 10 pregnant female mice groups at doses of 4.6, 9.2, or 18.5 mg/kg body weight (bw) of TI(I) acetate. | Curvature of the trunk, tail abnormalities, rotation defects in the forelimbs and hindlimbs. Delayed skeletal ossification. Effect dose: 4.6 mg/kg. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chiang, C.-K. The Impact of Thallium Exposure in Public Health and Molecular Toxicology: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4750. https://doi.org/10.3390/ijms25094750
Chang Y, Chiang C-K. The Impact of Thallium Exposure in Public Health and Molecular Toxicology: A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(9):4750. https://doi.org/10.3390/ijms25094750
Chicago/Turabian StyleChang, Yung, and Chih-Kang Chiang. 2024. "The Impact of Thallium Exposure in Public Health and Molecular Toxicology: A Comprehensive Review" International Journal of Molecular Sciences 25, no. 9: 4750. https://doi.org/10.3390/ijms25094750






