Anti-Biofilm Activity of Oleacein and Oleocanthal from Extra-Virgin Olive Oil toward Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Characterization of Clinical P. aeruginosa Strains
2.2. Antibacterial Activities of OOP, Oleacein, and Oleocanthal
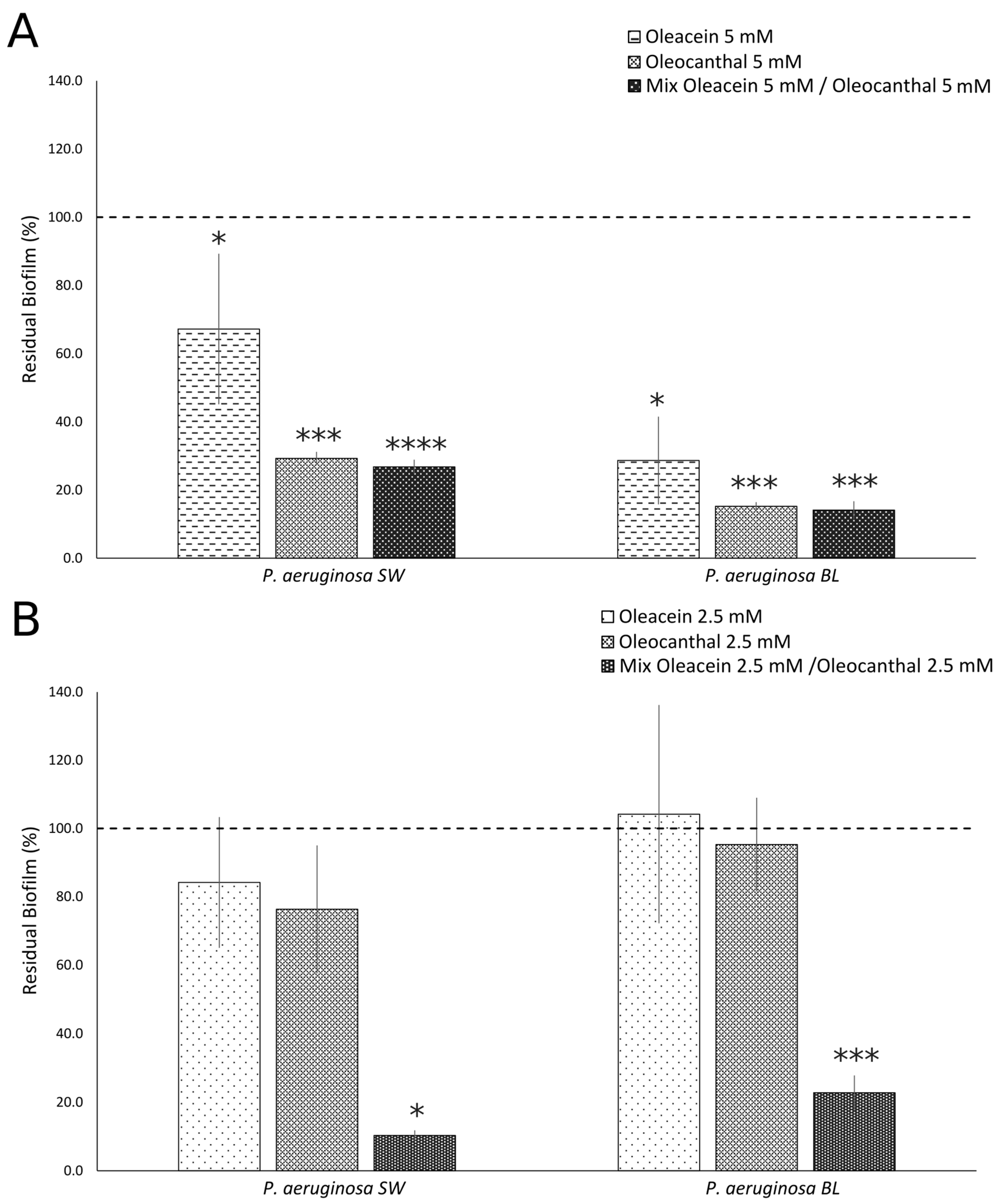
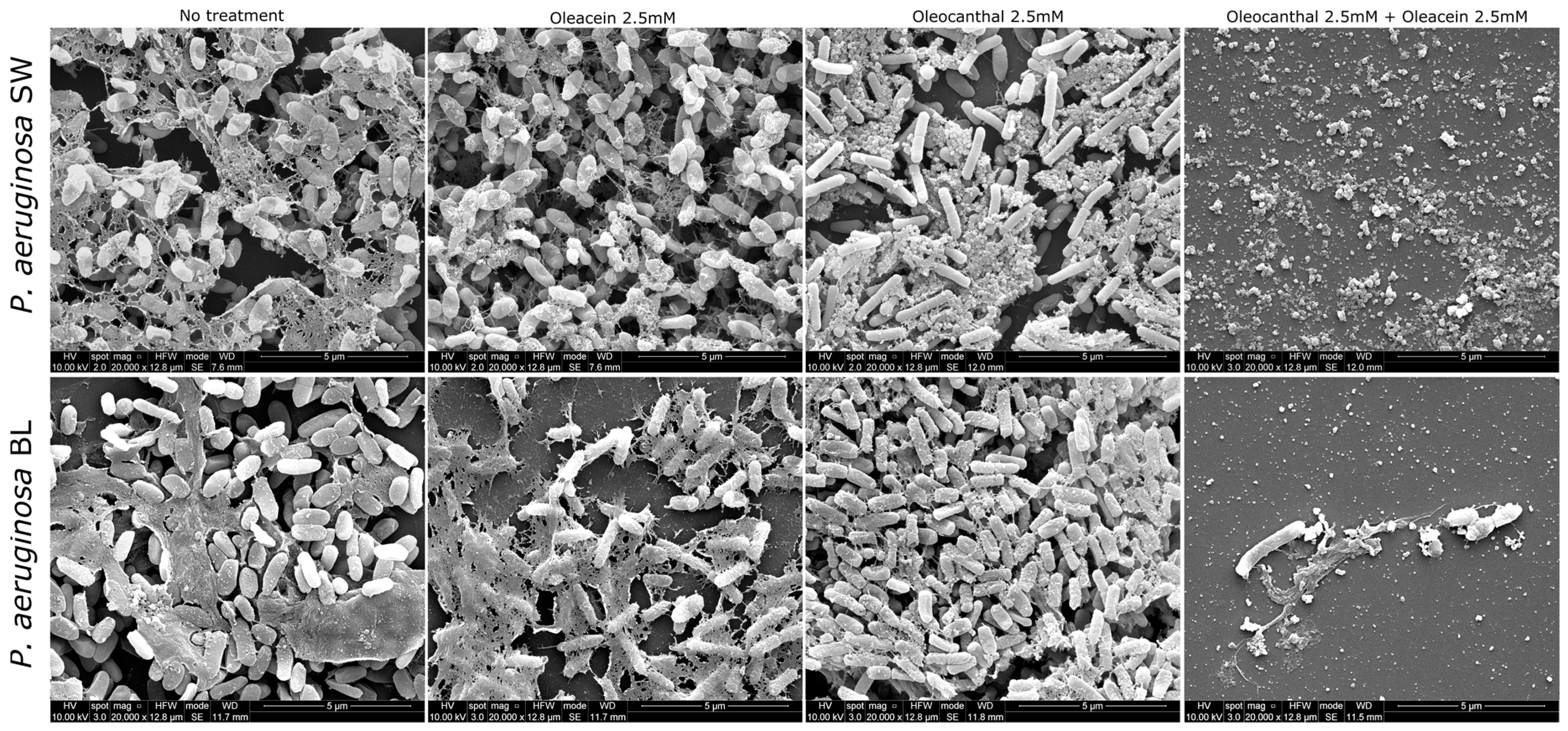
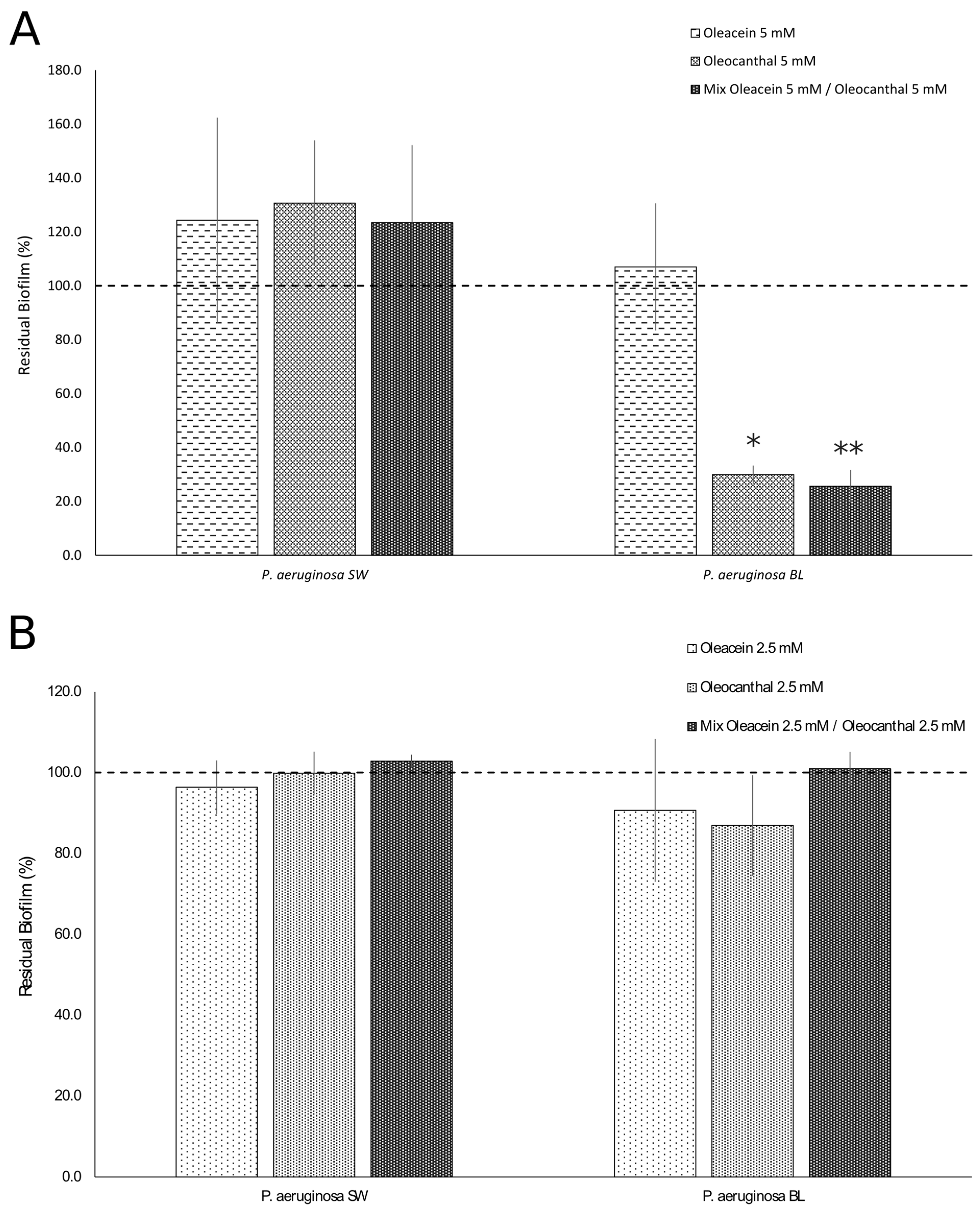
2.3. Anti-Biofilm Activities of OOP, Oleacein, and Oleocanthal
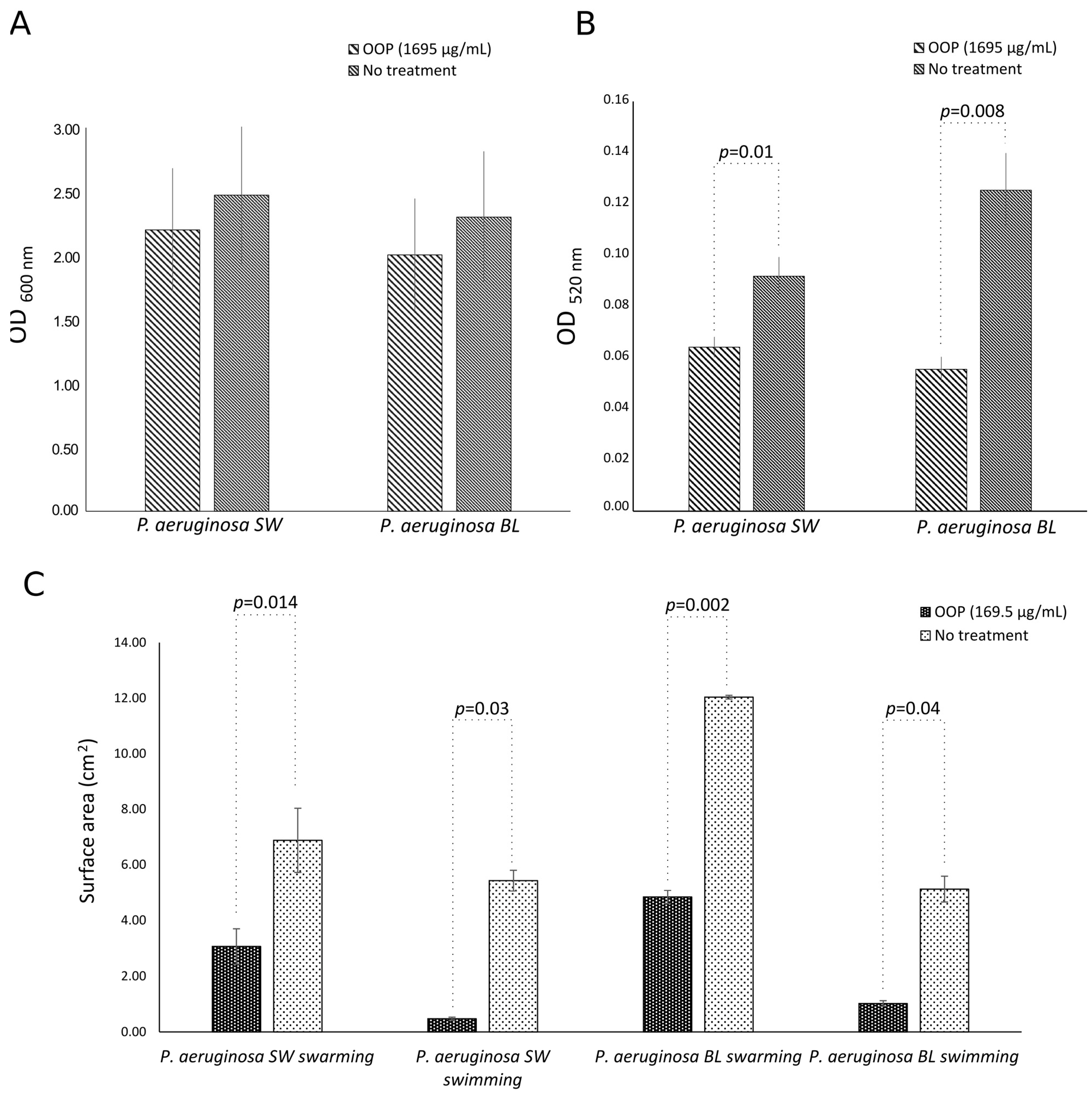
2.4. Anti-Virulence Activities of OOP, Oleacein, and Oleocanthal
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents
4.2. Bacterial Strains and Growth Conditions
4.3. Phenotypic Characterization of Clinical P. aeruginosa Strains
4.3.1. Antibiotic Susceptibility Assay
4.3.2. Biofilm Assay
4.3.3. Alginate Assay
4.3.4. Pyocyanin Assay
4.3.5. Motility Assay
4.4. Antibacterial Activities of OOP, Oleacein, and Oleocanthal
4.5. Anti-Biofilm Activities of OOP, Oleacein, and Oleocanthal
4.5.1. Biofilm Inhibition Assay
4.5.2. Mature Biofilm Eradication Assay
4.6. Anti-Virulence Activities of OOP, Oleacein, and Oleocanthal
4.6.1. Alginate Assay
4.6.2. Pyocyanin Assay
4.6.3. Motility Assay
4.7. Scanning Electron Microscopy
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. [Google Scholar]
- Filardo, S.; Roberto, M.; Di Risola, D.; Mosca, L.; Di Pietro, M.; Sessa, R. Olea Europaea L-Derived Secoiridoids: Beneficial Health Effects and Potential Therapeutic Approaches. Pharmacol. Ther. 2024, 254, 108595. [Google Scholar] [CrossRef]
- Di Pietro, M.; De Santis, F.; Schiavoni, G.; Filardo, S.; Sessa, R. Resveratrol in Chlamydia Pneumoniae-Induced Foam Cell Formation and Interleukin-17A Synthesis. J. Biol. Regul. Homeost. Agents 2013, 27, 509–518. [Google Scholar] [PubMed]
- Di Pietro, M.; Filardo, S.; De Santis, F.; Mastromarino, P.; Sessa, R. Chlamydia Pneumoniae and Oxidative Stress in Cardiovascular Disease: State of the Art and Prevention Strategies. Int. J. Mol. Sci. 2015, 16, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. Folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Federico, R.; Maggiore, A.; Fontana, M.; Boffi, A.; D’Erme, M.; Mosca, L. Green Route for the Isolation and Purification of Hyrdoxytyrosol, Tyrosol, Oleacein and Oleocanthal from Extra Virgin Olive Oil. Molecules 2020, 25, 3654. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Mattioli, R.; Bozzuto, G.; Molinari, A.; Mosca, L.; Sessa, R. Extra Virgin Olive Oil-Based Formulations: A “Green” Strategy against Chlamydia Trachomatis. Int. J. Mol. Sci. 2023, 24, 12701. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Sarabhai, S.; Sharma, P.; Capalash, N. Ellagic Acid Derivatives from Terminalia Chebula Retz. Downregulate the Expression of Quorum Sensing Genes to Attenuate Pseudomonas aeruginosa PAO1 Virulence. PLoS ONE 2013, 8, e53441. [Google Scholar] [CrossRef]
- Sathe, N.; Beech, P.; Croft, L.; Suphioglu, C.; Kapat, A.; Athan, E. Pseudomonas aeruginosa: Infections and Novel Approaches to Treatment “Knowing the Enemy” the Threat of Pseudomonas aeruginosa and Exploring Novel Approaches to Treatment. Infect. Med. 2023, 2, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef]
- Spoering, A.L.; Lewis, K. Biofilms and Planktonic Cells of Pseudomonas aeruginosa Have Similar Resistance to Killing by Antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The Role of Pyocyanin in Pseudomonas aeruginosa Infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.; Forbes, A.; Perkins, A.; Davey, A.; Chess-Williams, R.; Kiefel, M.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Khayat, M.T.; Abbas, H.A.; Ibrahim, T.S.; Elbaramawi, S.S.; Khayyat, A.N.; Alharbi, M.; Hegazy, W.A.H.; Yehia, F.A.A. Synergistic Benefits: Exploring the Anti-Virulence Effects of Metformin/Vildagliptin Antidiabetic Combination against Pseudomonas aeruginosa via Controlling Quorum Sensing Systems. Biomedicines 2023, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Bains, M.; Brazas, M.D.; Hancock, R.E.W. Swarming of Pseudomonas aeruginosa Is a Complex Adaptation Leading to Increased Production of Virulence Factors and Antibiotic Resistance. J. Bacteriol. 2008, 190, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Tranquilli, G.; Sessa, R. Biofilm in Genital Ecosystem: A Potential Risk Factor for Chlamydia Trachomatis Infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 1672109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A Major Ubiquitous Element of the Bacterial Biofilm Architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef]
- Bonincontro, G.; Scuderi, S.A.; Marino, A.; Simonetti, G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus Aureus, Pseudomonas aeruginosa, and Candida Spp. Pharmaceuticals 2023, 16, 1531. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- CDC National and State Healthcare-Associated Infections Progress Report. Available online: https://www.cdc.gov/hai/data/portal/progress-report.html (accessed on 6 February 2024).
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The Extracellular Matrix Protects Pseudomonas aeruginosa Biofilms by Limiting the Penetration of Tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of Exopolysaccharides in Pseudomonas aeruginosa Biofilm Formation and Architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Kutty, S.K.; Kumar, N.; Manefield, M. Pyocyanin Facilitates Extracellular DNA Binding to Pseudomonas aeruginosa Influencing Cell Surface Properties and Aggregation. PLoS ONE 2013, 8, e58299. [Google Scholar] [CrossRef] [PubMed]
- Kearns, D.B. A Field Guide to Bacterial Swarming Motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: http://www.eucast.org (accessed on 6 February 2024).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Papa, R.; Garzoli, S.; Vrenna, G.; Sabatino, M.; Sapienza, F.; Relucenti, M.; Donfrancesco, O.; Fiscarelli, E.V.; Artini, M.; Selan, L.; et al. Essential Oils Biofilm Modulation Activity, Chemical and Machine Learning Analysis. Application on Staphylococcus aureus Isolates from Cystic Fibrosis Patients. Int. J. Mol. Sci. 2020, 21, 9258. [Google Scholar] [CrossRef]
- Zheng, H.; Korendovych, I.V.; Luk, Y.-Y. Quantification of Alginate by Aggregation Induced by Calcium Ions and Fluorescent Polycations. Anal. Biochem. 2016, 492, 76–81. [Google Scholar] [CrossRef]
- Pejčić, M.; Stojanović-Radić, Z.; Genčić, M.; Dimitrijević, M.; Radulović, N. Anti-Virulence Potential of Basil and Sage Essential Oils: Inhibition of Biofilm Formation, Motility and Pyocyanin Production of Pseudomonas aeruginosa Isolates. Food Chem. Toxicol. 2020, 141, 111431. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guan, Y.; Zhou, J.; Sun, B.; Wang, Z.; Chen, H.; He, Z.; Jia, A. Phytochemicals from Camellia nitidissima Chi Flowers Reduce the Pyocyanin Production and Motility of Pseudomonas aeruginosa PAO1. Front. Microbiol. 2018, 8, 2640. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Mattioli, R.; Francioso, A.; Raponi, G.; Mosca, L.; Sessa, R. Extra Virgin Olive Oil-Based Green Formulations with Promising Antimicrobial Activity Against Drug-Resistant Isolates. Front. Pharmacol. 2022, 13, 885735. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 11th ed. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2019.


| P. aeruginosa Strain | Phenotype | Biofilm at 24 h OD 590 nm | Biofilm at 48 h OD 590 nm | Alginate OD 600 nm | Pyocyanin OD 520 nm | Swarming Area (cm2) | Swimming Area (cm2) |
|---|---|---|---|---|---|---|---|
| PA BL | Mucoid | 1.05 ± 0.59 | 0.81 ± 0.22 | 2.16 ± 0.33 | 0.12 ± 0.01 | 12.03 ± 0.06 | 5.11 ± 0.46 |
| PA SW | Non-Mucoid | 1.29 ± 0.87 | 1.10 ± 0.28 | 1.46 ± 0.17 | 0.09 ± 0.007 | 6.87 ± 1.16 | 5.42 ± 0.37 |
| p values | NS | NS | 0.048 | 0.037 | 0.005 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, M.; Filardo, S.; Mattioli, R.; Bozzuto, G.; Raponi, G.; Mosca, L.; Sessa, R. Anti-Biofilm Activity of Oleacein and Oleocanthal from Extra-Virgin Olive Oil toward Pseudomonas aeruginosa. Int. J. Mol. Sci. 2024, 25, 5051. https://doi.org/10.3390/ijms25095051
Di Pietro M, Filardo S, Mattioli R, Bozzuto G, Raponi G, Mosca L, Sessa R. Anti-Biofilm Activity of Oleacein and Oleocanthal from Extra-Virgin Olive Oil toward Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2024; 25(9):5051. https://doi.org/10.3390/ijms25095051
Chicago/Turabian StyleDi Pietro, Marisa, Simone Filardo, Roberto Mattioli, Giuseppina Bozzuto, Giammarco Raponi, Luciana Mosca, and Rosa Sessa. 2024. "Anti-Biofilm Activity of Oleacein and Oleocanthal from Extra-Virgin Olive Oil toward Pseudomonas aeruginosa" International Journal of Molecular Sciences 25, no. 9: 5051. https://doi.org/10.3390/ijms25095051





