Dietary Flavonoids Sensitize HeLa Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General
4.2. Cell culture
4.3. Cytotoxicity assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
4.4. LDH (lactate dehydrogenase) release assay
4.5. Detection of apoptosis
4.6. Statistical analysis
References
- Havesteen, B. The Flavonoids, a class of natural products oh high pharmacological potency. Biochem Pharmacol 1983, 32, 1141–1148, DOI 10.1016/0006-2952(83)90262-9; PubMed 6342623. [Google Scholar]
- Middleton, E. The Flavonoids. TIPS 1984, 5, 335–338. [Google Scholar]
- Middleton, E; Kandaswami, C; Theoharides, TC. The effect of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 2000, 52, 673–751, PubMed 11121513. [Google Scholar]
- Kanadaswami, C; Lee, LT; Lee, PP; Hwang, JJ; Ke, FC; Huang, YT; Lee, MT. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909, PubMed 16097445. [Google Scholar]
- Thomasset, SC; Berry, DP; Garcea, G; Marczylo, T; Steward, WP; Gescher, AJ. Dietary polyphenolic phytochemicals - promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer 2007, 120, 451–458, DOI 10.1002/ijc.22419; PubMed 17131309. [Google Scholar]
- Hemaisvarya, S; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother Res 2006, 20, 239–249, DOI 10.1002/ptr.1841; PubMed 16557604. [Google Scholar]
- Aggarwal, BB; Shishodia, S. Molecular targets of dietary agents of prevention and therapy of cancer. Biochem Pharmacol 2006, 71, 1397–1421, DOI 10.1016/j.bcp.2006.02.009; PubMed 16563357. [Google Scholar]
- Nozawa, F; Itami, A; Saruc, M; Kim, M; Standom, J; Picha, KS; Cowan, KH; Pour, PM. The combination of tumor necrosis factor related apoptosis inducing ligand (TRAIL/Apo2L) and genistein is effective in inhibiting pancreatic cancer growth. Pancreas 2004, 29, 45–52, DOI 10.1097/00006676-200407000-00055; PubMed 15211111. [Google Scholar]
- Shi, RX; Ong, CN; Shen, HM. Protein kinase C and X-linked inhibitor of apoptosis protein degradation contribute to the sensitization effect of luteolin on tumor necrosis factor-related apoptosis-inducing ligand induced apoptosis in cancer cells. Cancer Res 2005, 65, 7815–7823, DOI 10.1158/0008-5472.CAN-04-2749; PubMed 16140950. [Google Scholar]
- Horinaka, M; Yoshida, T; Shiraishi, T; Nakata, S; Wakada, M; Nakanishi, R; Nishino, H; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem Biophys Res Commun 2005, 333, 833–838, DOI 10.1016/j.bbrc.2005.05.179; PubMed 15963948. [Google Scholar]
- Horinaka, M; Yoshida, T; Shiraishi, T; Nakata, S; Wakada, M; Nakanishi, R; Nishino, H. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 2005, 24, 7180–7189, DOI 10.1038/sj.onc.1208874; PubMed 16007131. [Google Scholar]
- Kim, YH; Lee, YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem 2007, 100, 998–1009, PubMed 17031854. [Google Scholar]
- Chen, W; Wang, X; Zhuang, J; Lin, Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization oh TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis 2007, 4. ahead of print. [Google Scholar]
- Horinaka, M; Yoshida, T; Shiraishi, T; Nakata, S; Wakada, M; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther 2006, 5, 945–951, PubMed 16648565. [Google Scholar]
- Kuo, PL; Hsu, YL; Lin, TC; Lin, CC. The antiproliferative activity of prodelphidin B-2′ 3′-O-gallate from green tea leaf is through cell cycle arrest and Fas-mediated apoptotic pathway in A549 cells. Food Chem Toxicol 2005, 43, 315–323, PubMed 15621344. [Google Scholar]
- Zheng, PW; Chiang, LC; Lin, CC. Apigenin induces apoptyosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci 2005, 76, 1367–1379, PubMed 15670616. [Google Scholar]
- Pan, MH; Lai, PC; Hsu, PC; Wang, YJ. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem 2005, 53, 620–630, PubMed 15686411. [Google Scholar]
- Shi, RX; Ong, CN; Shen, HM. Luteolin sensitizes tumor necrosis factor-alpha apoptosis in human tumor cells. Oncogene 2004, 23, 7712–7721, PubMed 15334063. [Google Scholar]
- Kuo, PL; Lin, CC. Green tea constituent (−)-epigallocatechin-3-gallate Inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. Biomed Sci 2003, 10, 219–227. [Google Scholar]
- Ashkenazi, A; Pai, R; Fong, S; Leung, S; Lawrence, D; Marsters, S; Blackie, C; Chang, L; McMurtrey, AE; Hebert, A; DeForge, L; Koumenis, I; Lewis, D; Harris, D; Bussiere, J; Koeppen, H; Shahrokh, Z; Schwall, RH. Safety and antitumor activity of recombinant Apo2 ligand. J Clin Invest 1999, 104, 155–162, PubMed 10411544. [Google Scholar]
- Almasan, A; Ashkenazi, A. Apo2L/TRAIL: apoptosis signaling, biology and potential for cancer therapy. Cytokine Growth Factor Rev 2003, 14, 337–348, PubMed 12787570. [Google Scholar]
- Zhang, L; Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 2005, 12, 228–227, DOI 10.1038/sj.cgt.7700792; PubMed 15550937. [Google Scholar]
- Baud, V; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 2001, 11, 372–377, PubMed 11514191. [Google Scholar]
- Wajant, H; Pfizenmaier, K; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ 2003, 10, 45–65, PubMed 12655295. [Google Scholar]
- Lee, MW; Park, SC; Kim, JH; Kim, IK; Han, KS; Kim, KY; Lee, WB; Jung, YK; Kim, SS. The involvement of oxidative stress in tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in HeLa cells. Cancer Lett 2002, 182, 75–82, PubMed 12175526. [Google Scholar]
- Czuba, ZP; Krol, W. The importance of hydroxyl substituent in position 4′ in flavonoids for modulation of chemiluminescence generated by an enzymatic system (horseradish peroxidase – luminol – hydrogen peroxide). Curr Top Biophys 1996, 20S, 38–41. [Google Scholar]
- Shakibaei, M; Schulze-Tanzil, G; Takada, Y; Aggarwal, BB. Redox regulation of apoptosis by member of the TNF superfamily. Antioxid Redox Signal 2005, 7, 482–496, PubMed 15706096. [Google Scholar]
- Shen, HM; Pervaiz, S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J 2006, 20, 1589–1598, PubMed 16873882. [Google Scholar]
- Cell proliferation kit I (MTT), Instruction manual Version 3; Roche Applied Science: Germany, 2003.
- Cytotoxicity detection kit (LDH), Instruction manual Version 5; Roche Applied Science: Germany, 2004.
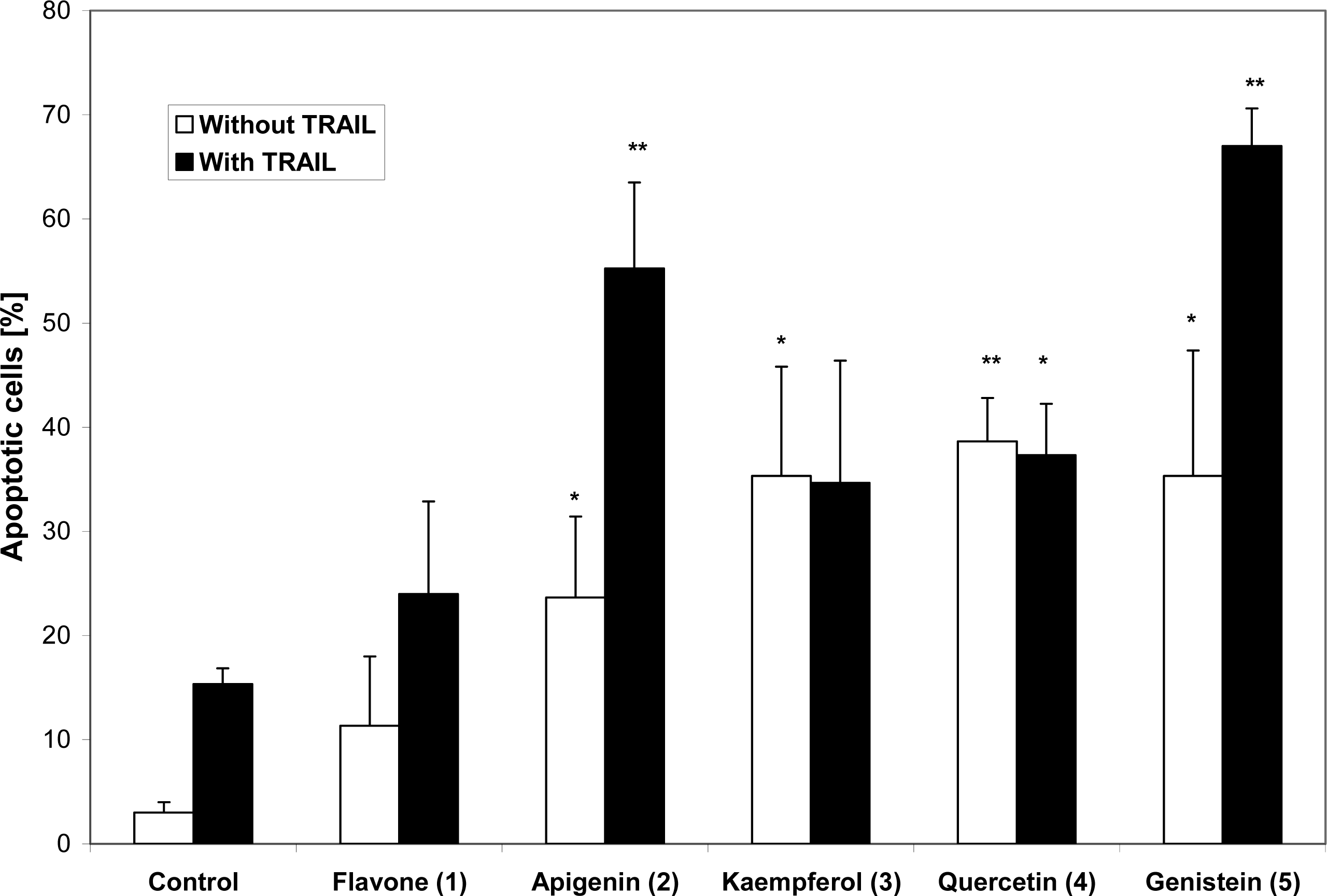
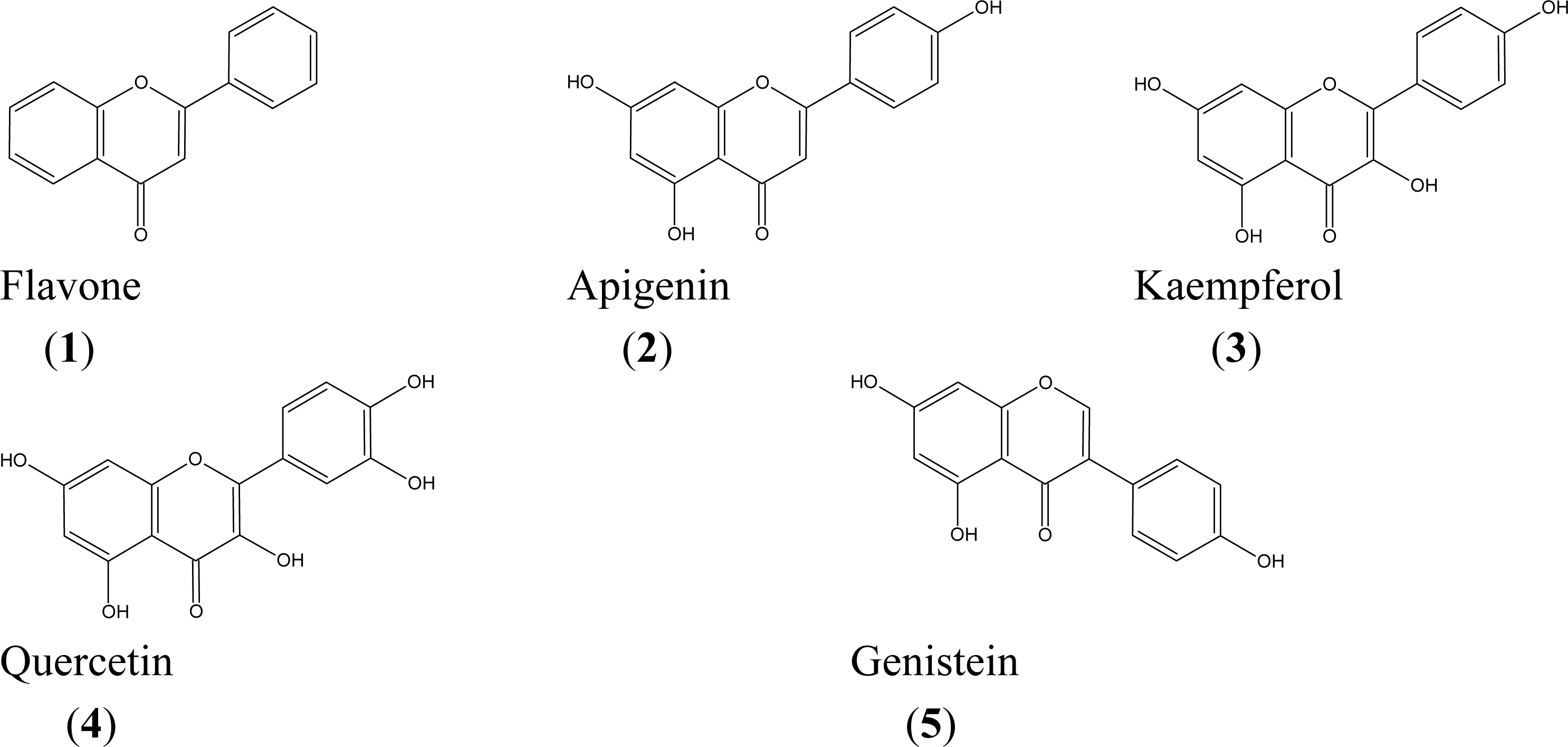
| Compound | IC50 (μM)a | |
|---|---|---|
| 1 | Flavone | 49.69±1.08 |
| 2 | Apigenin | 43.05±0.86 |
| 3 | Kaempferol | ndb |
| 4 | Quercetin | nd |
| 5 | Genistein | 49.64±2.43 |
Share and Cite
Szliszka, E.; Czuba, Z.P.; Jernas, K.; Król, W. Dietary Flavonoids Sensitize HeLa Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56-64. https://doi.org/10.3390/ijms9010056
Szliszka E, Czuba ZP, Jernas K, Król W. Dietary Flavonoids Sensitize HeLa Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). International Journal of Molecular Sciences. 2008; 9(1):56-64. https://doi.org/10.3390/ijms9010056
Chicago/Turabian StyleSzliszka, Ewelina, Zenon P. Czuba, Katarzyna Jernas, and Wojciech Król. 2008. "Dietary Flavonoids Sensitize HeLa Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)" International Journal of Molecular Sciences 9, no. 1: 56-64. https://doi.org/10.3390/ijms9010056




