The Effect of the Argon Carrier Gas in the Multiphoton Dissociation-Ionization of Tetracene
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
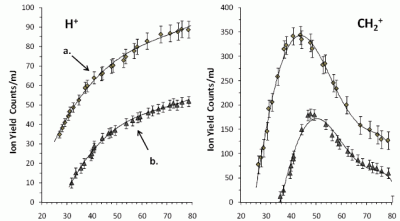
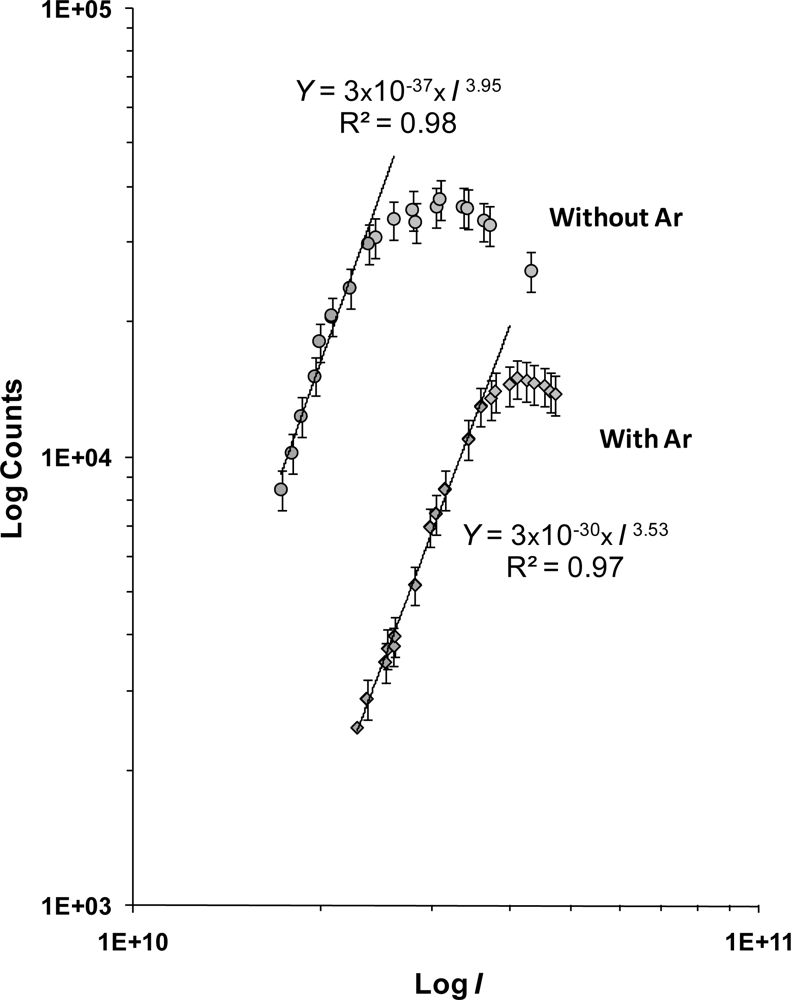
3.1. Photodissociation/photoionization of Tetracene without CG
3.2. Effect of the argon CG
4. Conclusions
Acknowledgments
References
- Disdier, B; Arfi, C; Pastor, J; Pauli, AM; Portugal, H. Analysis by GC-MS of monocyclic and polycyclic aromatic hydrocarbons in thermolysed waste products. Analusis 1999, 27, 235–240. [Google Scholar]
- Lesellier, E. Analysis of polycyclic aromatic hydrocarbons by supercritical fluid chromatography (SFC). Analusis 1999, 27, 241–248. [Google Scholar]
- van der Zwet, GP; Allamandola, LJ. Polycyclic aromatic hydrocarbons and the diffuse interstellar bands. Astron. Astrophys 1985, 146, 76–80. [Google Scholar]
- Allamandola, LJ; Tielens, AGGM; Barker, JR. Interstellar polycyclic aromatic hydrocarbons: the infrared emission bands, the excitation-emission mechanism and the astrophysical implications. Ap. J. Suppl. 1989, 71, 733–775. [Google Scholar]
- Schlemmer, S; Cook, DJ; Harrison, JA; Wurfel, B; Chapman, W; Saykally, RJ. The unidentified interstellar infrared bands: PAHs as carriers? Science 1994, 265, 1686–1689. [Google Scholar]
- Hudgins, DM; Allamandola, LJ. Infrared spectroscopy of matrix-isolated polycyclic aromatic hydrocarbon cations 4: The tetracyclic PAH isomers chrysene and 1,2-benzanthracene. J. Phys. Chem. A 1997, 101, 3472–3477. [Google Scholar]
- Ekern, SP; Marshall, AG; Szczepanski, J; Vala, M. Photodissociation of gas-phase polycylic aromatic hydrocarbon cations. J. Phys. Chem. A 1998, 102, 3498–3504. [Google Scholar]
- Hudgins, DM; Sandford, SA. Infrared spectroscopy of matrix isolated polycyclic aromatic hydrocarbons. 1. PAHS containing two to four rings. J. Phys. Chem. A 1998, 102, 329–343. [Google Scholar]
- Ehrenfreund, P. Molecules on a Space Odyssey. Science 1999, 283, 1123–1124. [Google Scholar]
- Ledingham, KWD; Singhal, RP. High intensity laser mass spectrometry – A review. Int. J. Mass Spectrom. Ion Processes 1997, 163, 149–168. [Google Scholar]
- Castillejo, M; Martín, M; De Nalda, R. Nanosecond versus, picosecond molecular multiphoton fragmentation of ketene and cyclohexane. Laser Chem 1998, 18, 51–62. [Google Scholar]
- Ledingham, KWD; Kosmidis, C; Georgiou, S; Couris, S; Singhal, RP. A comparison of the femto-, pico- and nano-second multiphoton ionization and dissociation processes of NO2 at 248 and 496 nm. Chem. Phys. Lett 1995, 247, 555–563. [Google Scholar]
- Smith, DB; Miller, JC. Picosecond multiphoton ionization of molecular clusters. J. Chem. Phys 1989, 90, 5203–5205. [Google Scholar]
- DeWitt, MJ; Levis, RJ. Near-infrared femtosecond photoionization of cyclic aromatic hydrocarbons. J. Chem. Phys 1995, 102, 8670–8673. [Google Scholar]
- DeWitt, MJ; Levis, RJ. Concerning the ionization of large polyatomic molecules with intense ultrafast lasers. J. Chem. Phys 1999, 110, 11368–11375. [Google Scholar]
- Mejia-Ospino, E; Álvarez, I; Cisneros, C. OPO time of flight system for multiphoton ionization and dissociation studies. Rev. Mex. Fís. 2004, 50, 170–178. [Google Scholar]
- Karas, M. Time-of-flight mass spectrometer with improved resolution. J. Mass Spectrom 1997, 32, 1–11. [Google Scholar]
- Kingston, RG; Guilhaus, M; Brenton, AG; Beynon, JH. A structural investigation of [C6H6]2+ and [C6H6]+·ions involved in charge exchange reactions. Org. Mass Spectrom 1985, 20, 406–411. [Google Scholar]
- Wacks, ME. Electron-impact studies of aromatic hydrocarbons. II. Naphthacene, naphthaphene, chrysene, triphenylene, and pyrene. J. Chem. Phys 1964, 41, 1661–1667. [Google Scholar]
- Jochims, HW; Rühl, E; Baumgärtel, H; Tobita, S; Leach, S. VUV peaks in absorption spectra and photoion yield curves of polycyclic aromatic hydrocarbons and related compounds. Int. J. Mass Spectrom. Ion Processes 1997, 167, 35–53. [Google Scholar]
- Robson, L; Tasker, AD; Hankin, SM; Ledingham, KWD; Singhal, RP; Fang, X; McCanny, T; Kosmidis, C; Tzallas, P; Langley, AJ; Taday, PF; Divall, EJ. Analysis of polycyclic aromatic hydrocarbons (PAHs) using nanosecond laser desorption/femtosecond ionisation laser mass spectrometry (FLMS). 2000. [Google Scholar]
- D, KW; McKenna, P; McCanny, T; Kosmidis, C; Tzallas, P; Jaroszynski, DA; Jones, DR. Ionization and fragmentation dynamics of laser desorbed polycyclic aromatic hydrocarbons using femtosecond and nanosecond post-ionisation. Int. J. Mass Spectrom. 2002, 220, 69–85. [Google Scholar]
- Robson, L; Ledingham, KWD; Tasker, AD; McKenna, P; McCanny, T; Kosmidis, C; Jaroszynski, DA; Jones, DR; Issac, RC; Jamieson, S. Ionisation and fragmentation of polycyclic aromatic hydrocarbons by femtosecond laser pulses at wavelengths resonant with cation transitions. Chem. Phys. Lett. 2002, 360, 382–389. [Google Scholar]
- Kosmidis, C; Ledingham, KWD; Kilic, HS; McCanny, T; Singhal, RP; Langley, AJ; Shaikh, W. On the fragmentation of nitrobenzene and nitrotoluenes induced by a femtosecond laser at 375 nm. J. Phys. Chem. A 1997, 101, 2264–2270. [Google Scholar]
- Faccineto, A; Thomson, K; Ziskind, M; Focsa, C. Coupling of desorption and photoionization processes in two-step laser mass spectrometry of polycyclic aromatic hydrocarbons. Appl. Phys. A 2008, 92, 969–974. [Google Scholar]
- Asvany, O; Savić, I; Schlemmer, S; Gerlich, D. Variable temperature ion trap studies of CH4++H2, HD and D2: Negative temperature dependence and significant isotope effect. Chem. Phys 2004, 298, 97–105. [Google Scholar]
- Olah, GA; Rasul, G. Comparison and search for CH53+ and CH64+ and their isoelectronic analogues BH52+ and BH63+1 1. J. Am. Chem. Soc 1996, 118, 12922–12924. [Google Scholar]
- Lammertsman, K; Barzaghi, M; Olah, GA; Pople, JA; Schleyer, P.v.P; Simonetta, M. Structure and stability of diprotonated methane, CH6+2. J. Am. Chem. Soc 1983, 105, 5258–5263. [Google Scholar]
- Ledingham, KWD; Smith, DJ; Singhal, RP; McCanny, T; Graham, P; Kilic, HS; Peng, WX; Langley, AJ; Taday, PF; Kosmidis, C. Multiply charged ions from aromatic molecules following irradiation in intense laser fields. J. Phys. Chem. A 1999, 103, 2952–2963. [Google Scholar]
- Robin, MB. Multiphoton fragmentation and ionization. Appl. Optics 1980, 19, 3941–3947. [Google Scholar]
- Troxler, Th; Leutwyler, S. Molecular dynamics and semiclassical electronic spectra of naphthalene-Arn clusters (n ≤ 4). J. Chem. Phys 1993, 99, 4363–4378. [Google Scholar]
- Troxler, Th; Leutwyler, S. Molecular spectra of naphthalene-Arn solvent clusters (n = 1–30). J. Chem. Phys 1991, 95, 4010–4023. [Google Scholar]
- Boesl, U; Weinkauf, R; Weickhardt, C; Schalg, EW. Laser ion sources for time-of-flight mass spectrometry. Int. J. Mass. Spec. Ion. Proc 1994, 131, 87–124. [Google Scholar]



| Ion | Without C.G.
| With C.G.
| ||
|---|---|---|---|---|
| n | n·hν, eV | n | n·hv, eV | |
| H+ | 2.23±0.22 | 7.78 | 2.02 ± 0.20 | 7.05 |
| C+ | 2.20±0.22 | 7.68 | 2.24 ± 0.22 | 7.82 |
| CH2+ | 5.74±0.57 | 20.03 | 3.60 ± 0.36 | 12.56 |
| CH3+ | --- | --- | 3.50 ± 0.35 | 12.22 |
| CH4+ | 4.40±0.44 | 15.36 | 3.36 ± 0.34 | 11.73 |
| CH5+ | 2.55±0..26 | 8.90 | 4.10 ± 0.41 | 14.31 |
| CH6+ | --- | --- | 3.02 ± 0.30 | 10.54 |
| C2H+ | 1.92±0.19 | 6.70 | --- | --- |
| C2H4+ | 3.72±0.37 | 12.98 | 1.63 ± 0.16 | 5.69 |
| C3+ | --- | --- | 3.11 ± 0.31 | 10.85 |
| C3H4+ | --- | --- | 4.20 ± 0.42 | 14.66 |
| C5H4+2 | --- | --- | 3.72 ± 0.37 | 12.98 |
| C7H8+2 | --- | --- | 5.10 ± 0.51 | 17.80 |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poveda, J.C.; San Román, A.; Guerrero, A.; Álvarez, I.; Cisneros, C. The Effect of the Argon Carrier Gas in the Multiphoton Dissociation-Ionization of Tetracene. Int. J. Mol. Sci. 2008, 9, 2003-2015. https://doi.org/10.3390/ijms9102003
Poveda JC, San Román A, Guerrero A, Álvarez I, Cisneros C. The Effect of the Argon Carrier Gas in the Multiphoton Dissociation-Ionization of Tetracene. International Journal of Molecular Sciences. 2008; 9(10):2003-2015. https://doi.org/10.3390/ijms9102003
Chicago/Turabian StylePoveda, Juan Carlos, Alejandro San Román, Alfonso Guerrero, Ignacio Álvarez, and Carmen Cisneros. 2008. "The Effect of the Argon Carrier Gas in the Multiphoton Dissociation-Ionization of Tetracene" International Journal of Molecular Sciences 9, no. 10: 2003-2015. https://doi.org/10.3390/ijms9102003