Aspergillus parasiticus crzA, Which Encodes Calcineurin Response Zinc-Finger Protein, Is Required for Aflatoxin Production under Calcium Stress
Abstract
:1. Introduction
2. Results
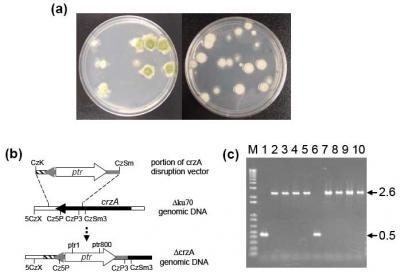
2.1. Disruption of crzA in morphologically different A. parasiticus strains
2.2. Effects of metal ions, pH and light on vegetative growth and asexual development
2.3. Calcium supplementation on relative expression levels of calcium-transporting genes
2.4. Calcium supplementation on aflatoxin or OMST production and on relative expression levels of aflatoxin biosynthesis genes
3. Discussion
4. Experimental Section
4.1. Fungal strains
4.2. In silico identification of A. flavus crzA gene
4.3. Deletion of crzA in A. parasiticus strains
4.4. Determination of colony growth
4.5. Estimation of conidial production by RH ku70 and the RH crzA mutants
4.6. Effects of ions and pH on the crzA mutants
4.7. Semi-quantitative thin layer chromatography (TLC) analysis of aflatoxins and OMST
4.8. Determination of relative expression levels of plasma membrane (P-type) calcium-transporting ATPase gene homologues in BN9ΔcrzA and RHΔcrzA mutants
4.9. Determination of relative expression levels of aflatoxin biosynthesis genes in the BN9 crzA and RH crzA mutants
5. Conclusions
Acknowledgments
References
- Kurtzman, CP; Horn, BW; Hesseltine, CW. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Leeuwenhoek 1987, 53, 147–158. [Google Scholar]
- Horn, BW. Biodiversity of Aspergillus section Flavi in the United States: a review. Food. Addit. Contam. 2007, 24, 1088–1101. [Google Scholar]
- Horn, BW; Greene, RL; Sobolev, VS; Dorner, JW; Powell, JH; Layton, RC. Association of morphology and mycotoxin production with vegetative compatibility groups in Aspergillus flavus, A. parasiticus, and A. tamarii. Mycologia 1996, 88, 574–587. [Google Scholar]
- Calvo, A.M; Bok, J; Brooks, W; Keller, NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol 2004, 70, 4733–4739. [Google Scholar]
- Hicks, JK; Yu, JH; Keller, NP; Adams, TH. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 1997, 16, 4916–4923. [Google Scholar]
- Roze, LV; Arthur, AE; Hong, SY; Chanda, A; Linz, JE. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol. Microbiol. 2007, 66, 713–726. [Google Scholar]
- Bennett, JW; Rubin, PL; Lee, LS; Chen, PN. Influence of trace elements and nitrogen sources on versicolorin production by a mutant strain of. Aspergillus parasiticus. Mycopathologia 1979, 69, 161–166. [Google Scholar]
- Payne, GA; Hagler, WMJ. Effect of specific amino acids on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus in defined media. Appl. Environ. Microbiol. 1983, 46, 805–812. [Google Scholar]
- Reddy, TV; Viswanathan, L; Venkitasubramanian, TA. Factors affecting aflatoxin production by Aspergillus parasiticus in a chemically defined medium. J. Gen. Microbiol. 1979, 114, 409–413. [Google Scholar]
- Wilkinson, JR; Yu, J; Abbas, HK; Scheffler, BE; Kim, HS; Nierman, WC; Bhatnagar, D; Cleveland, TE. Aflatoxin formation and gene expression in response to carbon source media shift in Aspergillus parasiticus. Food. Addit. Contam. 2007, 24, 1051–1060. [Google Scholar]
- Wilkinson, JR; Yu, J; Bland, JM; Nierman, WC; Bhatnagar, D; Cleveland, TE. Amino acid supplementation reveals differential regulation of aflatoxin biosynthesis in Aspergillus flavus NRRL 3357 and Aspergillus parasiticus SRRC 143. Appl. Microbiol. Biotechnol. 2007, 74, 1308–1319. [Google Scholar]
- Cuero, R; Ouellet, T; Yu, J; Mogongwa, N. Metal ion enhancement of fungal growth, gene expression and aflatoxin synthesis in Aspergillus flavus: RT-PCR characterization. J. Appl. Microbiol. 2003, 94, 953–961. [Google Scholar]
- Lillehoj, EB; Garcia, WJ; Lambrow, M. Aspergillus flavus infection and aflatoxin production in corn: influence of trace elements. Appl. Microbiol. 1974, 28, 763–767. [Google Scholar]
- Marsh, PB; Simpson, ME; Trucksess, MW. Effects of trace metals on the production of aflatoxins by Aspergillus parasiticus. Appl. Microbiol. 1975, 30, 52–57. [Google Scholar]
- Tiwari, RP; Mittal, V; Bhalla, TC; Saini, SS; Singh, G; Vadehra, DV. Effect of metal ions on aflatoxin production by Aspergillus parasiticus. Folia Microbiol. (Praha) 1986, 31, 124–128. [Google Scholar]
- Stie, J; Fox, D. Calcineurin regulation in fungi and beyond. Eukaryot. Cell 2008, 7, 177–186. [Google Scholar]
- Maggon, KK; Gupta, SK; Venkitasubramanian, TA. Biosynthesis of aflatoxins. Bacteriol. Rev. 1977, 41, 822–855. [Google Scholar]
- Rao Praveen, J; Subramanyam, C. Requirement of Ca2+ for aflatoxin production: inhibitory effect of Ca2+ channel blockers on aflatoxin production by Aspergillus parasiticus NRRL 2999. Lett. Appl. Microbiol. 1999, 28, 85–88. [Google Scholar]
- Joseph, JD; Means, AR. Calcium binding is required for calmodulin function in Aspergillus nidulans. Eukaryot. Cell 2002, 1, 119–125. [Google Scholar]
- Kraus, PR; Heitman, J. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 2003, 311, 1151–115. [Google Scholar]
- Rakhilin, SV; Olson, PA; Nishi, A; Starkova, NN; Fienberg, AA; Nairn, AC; Surmeier, DJ; Greengard, P. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science 2004, 306, 698–701. [Google Scholar]
- Cyert, MS. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar]
- da Silva Ferreira, ME; Heinekamp, T; Hartl, A; Brakhage, AA; Semighini, CP; Harris, SD; Savoldi, M; de Gouvea, PF; de Souza Goldman, MH; Goldman, GH. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 2007, 44, 219–230. [Google Scholar]
- Karababa, M; Valentino, E; Pardini, G; Coste, AT; Bille, J; Sanglard, D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol 2006, 59, 1429–1451. [Google Scholar]
- Stathopoulos, AM; Cyert, MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997, 11, 3432–3444. [Google Scholar]
- Hernandez-Lopez, MJ; Panadero, J; Prieto, JA; Randez-Gil, F. Regulation of salt tolerance by Torulaspora delbrueckii calcineurin target Crz1p. Eukaryot. Cell 2006, 5, 469–479. [Google Scholar]
- Schumacher, J; de Larrinoa, IF; Tudzynski, B. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 2008, 7, 584–601. [Google Scholar]
- Soriani, FM; Malavazi, I; da Silva Ferreira, ME; Savoldi, M; Von Zeska Kress, MR; de Souza Goldman, MH; Loss, O; Bignell, E; Goldman, GH. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 2008, 67, 1274–1291. [Google Scholar]
- Cramer, RA, Jr; Perfect, BZ; Pinchai, N; Park, S; Perlin, DS; Asfaw, YG; Heitman, J; Perfect, JR; Steinbach, WJ. The calcineurin target CrzA regulates conidial germination, hyphal growth and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 2008, 7, 1085–1097. [Google Scholar]
- Greene, V; Cao, H; Schanne, FA; Bartelt, DC. Oxidative stress-induced calcium signalling in Aspergillus nidulans. Cell Signal 2002, 14, 437–443. [Google Scholar]
- Price, A.H; Taylor, A; Ripley, SJ; Griffiths, A; Trewavas, AJ; Knight, MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 1994, 6, 1301–1310. [Google Scholar]
- Kim, JH; Yu, J; Mahoney, N; Chan, KL; Molyneux, RJ; Varga, J; Bhatnagar, D; Cleveland, TE; Nierman, WC; Campbell, BC. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar]
- Narasaiah, KV; Sashidhar, RB; Subramanyam, C. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar]
- Reverberi, M; Fabbri, AA; Zjalic, S; Ricelli, A; Punelli, F; Fanelli, C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005, 69, 207–215. [Google Scholar]
- Spielvogel, A; Findon, H; Arst, HN, Jr; Araujo-Bazan, L; Hernandez-Ortiz, P; Stahl, U; Meyer, V; Espeso, EA. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homeostasis and detoxification in Aspergillus nidulans. Biochem. J. 2008, 414, 419–429. [Google Scholar]
- Chang, P-K. A highly efficient gene-targeting system for Aspergillus parasiticus. Lett. Appl. Microbiol 2008, 46, 587–592. [Google Scholar]
- Ehrlich, KC; Scharfenstein, LL, Jr; Montalbano, BG; Chang, P-K. Are the genes nadA and norB involved in formation of aflatoxin G1? Int. J. Mol. Sci. 2008, 9, 1719–1729. [Google Scholar]
- Mooney, JL; Yager, LN. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990, 4, 1473–1482. [Google Scholar]
- Kafer, E. Origins of translocations in Aspergillus nidulans. Genetics 1965, 52, 217–232. [Google Scholar]
- Stinnett, SM; Espeso, EA; Cobeno, L; Araujo-Bazan, L; Calvo, AM. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 2007, 63, 242–255. [Google Scholar]
- Bennett, JW; Fernholz, FA; Lee, LS. Effect of light on aflatoxins, anthraquinones, and sclerotia in Aspergillus flavus and A. parasiticus. Mycologia 1978, 70, 104–116. [Google Scholar]
- Rai, JN; Tewari, JP; Sinha, AK. Effect of environmental conditions on sclerotia and cleistothecia production in Aspergillus. Mycopathol. Mycol. Appl. 1967, 31, 209–224. [Google Scholar]
- Geiser, DM; Timberlake, WE; Arnold, ML. Loss of meiosis inAspergillus. Mol. Biol. Evol. 1996, 13, 809–817. [Google Scholar]
- Cary, JW; O'Brian, GR; Nielsen, DM; Nierman, W; Harris-Coward, P; Yu, J; Bhatnagar, D; Cleveland, TE; Payne, GA; Calvo, AM. Elucidation of veA-dependent genes associated with aflatoxin and sclerotial production in Aspergillus flavus by functional genomics. Appl. Microbiol. Biotechnol. 2007, 76, 1107–1118. [Google Scholar]
- Paschen, W; Mengesdorf, T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005, 38, 409–415. [Google Scholar]
- Nicotera, P; Petersen, OH; Melino, G; Verkhratsky, A. Janus a god with two faces: Death and survival utilize same mechanisms conserved by evolution. Cell Death Differ. 2007, 14, 1235–1236. [Google Scholar]
- Caspersen, C; Pedersen, PS; Treiman, M. The sarco/endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible protein. J. Biol. Chem. 2000, 275, 22363–22372. [Google Scholar]
- Fan, W; Idnurm, A; Breger, J; Mylonakis, E; Heitman, J. Eca1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 2007, 75, 3394–3405. [Google Scholar]
- Rao Praveen, J; Subramanyam, C. Calmodulin mediated activation of acetyl-CoA carboxylase during aflatoxin production by Aspergillus parasiticus. Lett. Appl. Microbiol. 2000, 30, 277–281. [Google Scholar]
- Jayashree, T; Praveen Rao, J; Subramanyam, C. Regulation of aflatoxin production by Ca2+/calmodulin-dependent protein phosphorylation and dephosphorylation. FEMS Microbiol. Lett. 2000, 183, 215–219. [Google Scholar]
- Lee, EGH; Townsley, PM; Walden, CC. Effect of bivalent metals on the production of aflatoxin in submerged cultures. J. Food Sci. 1964, 31, 208–211. [Google Scholar]
- Mateles, RI; Adye, JC. Production of aflatoxin in submerged culture. Appl. Microbiol. 1965, 13, 208–211. [Google Scholar]
- Chung, K-R. Involvement of calcium/calmodulin signaling in cercosporin toxin biosynthesis by Cercospora nicotianae. Appl. Environ. Microbiol. 2003, 69, 1187–1196. [Google Scholar]
- Yoshimoto, H; Saltsman, K; Gasch, AP; Li, HX; Ogawa, N; Botstein, D; Brown, PO; Cyert, MS. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 31079–31088. [Google Scholar]
- Guzman-de-Pena, D; Ruiz-Herrera, J. Relationship between aflatoxin biosynthesis and sporulation in Aspergillus parasiticus. Fungal Genet. Biol. 198–205.
- Wilkinson, H; Ramaswamy, A; Sim, SC; Keller, NP. Increased conidiation associated with progression along the sterigmatocystin biosynthetic pathway. Mycologia 2004, 96, 1190–1198. [Google Scholar]
- Chang, P-K. TheAspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genom. 2003, 268, 711–719. [Google Scholar]




| Strain | Gene a | Parent | Mutant 1 | Mutant 2 |
|---|---|---|---|---|
| BN9
| 2893 | 4.4b | 1.4 | 1.3 |
| 9335 | 2.8 | 1.0 | 0.9 | |
| 10917
| 1.3
| 0.8
| 0.7
| |
| RH | 2893 | 2.9 | 1.2 | 0.7 |
| 9335 | 0.4 | 0.4 | 0.5 | |
| 10917 | 0.3 | 0.3 | 0.3 |
| Strain | Gene | Parent | Mutant 1 | Mutant 2 | |||
|---|---|---|---|---|---|---|---|
| 48h
| 72h
| 48h
| 72h
| 48h
| 72h
| ||
| BN9
| aflR | 0.78a | 0.32 | 0.16 | 0.06 | 0.34 | 0.35 |
| nor1 | 0.58 | 0.21 | 0.05 | 0.02 | 0.15 | 0.10 | |
| ver1 | 0.60 | 0.17 | 0.09 | 0.01 | 0.13 | 0.12 | |
| omtA | 0.53
| 0.41
| 0.05
| 0.01
| 0.14
| 0.17
| |
| RH | aflR | 0.76 | 0.44 | 0.89 | 0.15 | 0.33 | 0.13 |
| nor1 | 0.48 | 0.56 | 0.24 | 0.05 | 0.19 | 0.06 | |
| ver1 | 0.57 | 0.99 | 0.22 | 0.08 | 0.14 | 0.02 | |
| omtA | 0.41 | 0.79 | 0.20 | 0.07 | 0.31 | 0.28 | |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, P.-K. Aspergillus parasiticus crzA, Which Encodes Calcineurin Response Zinc-Finger Protein, Is Required for Aflatoxin Production under Calcium Stress. Int. J. Mol. Sci. 2008, 9, 2027-2043. https://doi.org/10.3390/ijms9102027
Chang P-K. Aspergillus parasiticus crzA, Which Encodes Calcineurin Response Zinc-Finger Protein, Is Required for Aflatoxin Production under Calcium Stress. International Journal of Molecular Sciences. 2008; 9(10):2027-2043. https://doi.org/10.3390/ijms9102027
Chicago/Turabian StyleChang, Perng-Kuang. 2008. "Aspergillus parasiticus crzA, Which Encodes Calcineurin Response Zinc-Finger Protein, Is Required for Aflatoxin Production under Calcium Stress" International Journal of Molecular Sciences 9, no. 10: 2027-2043. https://doi.org/10.3390/ijms9102027