T-2 Toxin-induced Toxicity in Pregnant Mice and Rats
Abstract
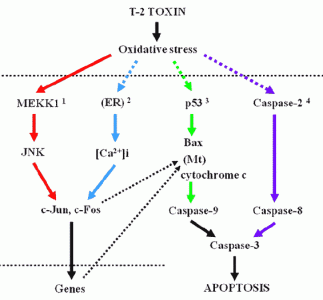
:1. Introduction
2. Maternal toxicity
3. Fetal toxicity
4. Relationship between maternal and fetal toxicities
5. Conclusions
Acknowledgments
References
- Desjardins, AE; Hohn, TM; McCormic, SP. Trichothecene Biosynthesis in Fusarium Species: Chemistry, Genetics, and Significance. Microbiol. Rev 1993, 57, 595–604. [Google Scholar]
- Nelson, PE; Dignani, MC; Anaissie, EJ. Taxonomy, Biology, and Clinical Aspects of Fusarium Species. Clin. Microbiol. Rev 1994, 7, 479–504. [Google Scholar]
- Joffe, AZ. Foodborne Diseases: Alimentary Toxic Aleukia. In Handbook of Foodborne Diseases of Biological Origin; Rochcigle, M, Ed.; CRC Press: Boca Raton, FL, 1983; pp. 353–495. [Google Scholar]
- Saito, M; Ohtsubo, K. Trichothecene Toxins of Fusarium Species. In Mycotoxins; Purchase, IFH, Ed.; Elsevier Scientific Publication: New York, 1977; pp. 264–280. [Google Scholar]
- Ueno, Y; Ishii, K; Saki, K; Kanadera, K; Tsunoda, S; Tanoka, H; Enomoto, M. Toxicological Approaches to the Metabolites of Fusaria. IV. Microbial Survey on “Bean-Hulls Poisoning of Horses” with the Isolation of Toxic Trichothecenes, Neosonaniol and T-2 Toxin of Fusarium solani M-1-1. Jpn. J. Exp. Med 1972, 42, 187–203. [Google Scholar]
- Shifrin, VI; Anderson, P. Trichothecene Mycotoxins Trigger a Ribotoxic Stress Response that Activates c-Jun N-Terminal Kinase and p38 Mitogen-Activated Protein Kinase and Induces Apoptosis. J. Biol. Chem 1999, 274, 13985–13992. [Google Scholar]
- Bennet, JW; Klich, M. Mycotoxins. Clin. Microviol. Rev 2003, 16, 497–516. [Google Scholar]
- Eriksen, GS; Petterson, H. Toxicological Evaluation of Trichothecenes in Animal Feed. Anim. Feed Sci. Technol 2004, 114, 205–239. [Google Scholar]
- Thompson, WL; Wannemacher, RW, Jr. In Vivo Effects of T-2 Mycotoxin on Synthesis of Protein and DNA in Rat Tissues. Toxicol. Appl. Pharmacol 1990, 105, 483–491. [Google Scholar]
- Chang, IM; Mar, WC. Effect of T-2 Toxin on Lipid Peroxidation in Rats: Elevation of Conjugated Diene Formation. Toxicol. Lett 1988, 40, 275–280. [Google Scholar]
- Eriksen, GS; Petterson, H; Lund, H. Comparative Cytotoxicity of Deoxynivalenol, Nivalenol, Theiracetylated Derivatives and De-Epoxy Metabolites. Food Chem. Toxicol 2004, 42, 619–624. [Google Scholar]
- Donal, V; Jezkova, A; Jun, D; Kuca, K. Metabolic Pathways of T-2 Toxin. Curr. Drug Metab 2008, 9, 77–82. [Google Scholar]
- Stanford, GK; Hood, RD; Haynes, AW. Effects of Prenatal Administration of T-2 Toxin to Mice. Res. Commu. Chem. Pathol. Pharmacol 1975, 10, 743–746. [Google Scholar]
- Williams, PP. Effects of T-2 Mycotoxin on Gastrointestinal Tissues: A View of in Vivo and in Vitro Models. Arch. Environ. Contam. Toxicol 1989, 18, 374–387. [Google Scholar]
- IARC. Toxins derived from Fusarium sporotrichioides: T-2 Toxin. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, 1993; pp. 467–488. [Google Scholar]
- Sharma, RP. Immunotoxicity of Mycotoxins. J. Dairy Sci 1993, 76, 892–897. [Google Scholar]
- Magnuson, BA; Schiefer, HB; Hancock, DS; Bhatti, AR. Cardiovascular Effects of Mycotoxin T-2 after Topical Application in Rats. Can. J. Physiol. Pharmacol 1987, 65, 799–802. [Google Scholar]
- Lutsky, I; Mor, N. Experimental Alimentary Toxic Aleukia in Cats. Lab. Anim. Sci 1981, 31, 43–47. [Google Scholar]
- Quiroga, MA; Itagaki, S; Doi, K. Early Ultrastructural Changes of Thymocytes in T-2 Toxicated Mice. J. Toxicol. Pathol 1993, 6, 109–112. [Google Scholar]
- Shinozuka, J; Li, G; Kiatipattanasakul, W; Uetsuka, K; Nakayama, H; Doi, K. T-2 Toxin-induced Apoptosis in Lymphoid Organs of Mice. Exp. Toxicol. Pathol 1997, 49, 387–392. [Google Scholar]
- Li, G; Shinozuka, J; Uetsuka, K; Nakayama, H; Doi, K. T-2 Toxin-induced Apoptosis in Peyer’s Patches of Mice. J. Toxicol. Pathol 1997, 10, 59–61. [Google Scholar]
- Shinozuka, J; Suzuki, M; Noguchi, N; Sugimoto, T; Uetsuka, K; Nakayama, H; Doi, K. T-2 Toxin-induced Apoptosis in Hematopoietic Tissues of Mice. Toxicol. Pathol 1998, 26, 674–681. [Google Scholar]
- Li, G; Shinozuka, J; Uetsuka, K; Nakayama, H; Doi, K. T- 2 Toxin- induced Apoptosis in Intestinal Crypt Epithelial Cells of Mice. Exp. Toxico. Pathol 1997, 49, 447–450. [Google Scholar]
- Albarenque, SM; Shinozuka, J; Iwamoto, S; Nakayama, H; Doi, K. T-2 Toxin-induced Acute Skin Lesions in Wistar-Derived Hypotrichotic WBN/ILA-Ht Rats. Histol. Histopathol 1999, 14, 337–342. [Google Scholar]
- Shinozuka, J; Miwa, S; Fujimura, H; Toriumi, W; Doi, K. Hepatotoxicity of T-2 Toxin, Trichothecene Mycotoxin. In New Strategies for Mycotoxin research in Asia (Proceedings of ISMYCO Bangkok ’06); Kumagai, S, Ed.; Japanese Association of Mycotoxicology: Tokyo, 2007; pp. 62–66. [Google Scholar]
- Albarenque, SM; Shinozuka, J; Suzuki, K; Nakayama, H; Doi, K. Kinetics and Distribution of Transforming Growth Factor (TGF)-β1 mRNA in the Dorsal Skin of Hypotrichotic WBN/ILA-Ht Rats Following Topical Application of T-2 Toxin. Exp. Toxicol. Pathol 2000, 52, 297–301. [Google Scholar]
- Albarenque, SM; Suzuki, K; Nakayama, H; Doi, K. Kinetics of Cytokines mRNAs Expression in the Dorsal Skin of WBN/ILA-Ht Rats Following Topical Application of T-2 Toxin. Exp. Toxicol. Pathol 2001, 53, 271–274. [Google Scholar]
- Albarenque, SM; Suzuki, K; Shinozuka, J; Nakayama, H; Doi, K. Kinetics of Apoptosis-related Genes mRNAs Expression in the Dorsal Skin of HypotrichoticWBN/ILA-Ht Rats after Topical Application of T-2 Toxin. Exp. Toxicol. Pathol 2001, 52, 553–556. [Google Scholar]
- Albarenque, SM; Doi, K. T-2 Toxin-induced Apoptosis in Rat Keratinocyte Primary Cultures. Exp. Mol. Pathol 2005, 78, 144–149. [Google Scholar]
- Lafarge-Frayssinet, C; Chakor, K; Lafont, P; Frayssinet, C. Transplacental Transfer of T2 Toxin: Pathological Effect. J. Environ. Pathol. Toxicol. Oncol 1990, 10, 64–68. [Google Scholar]
- Schoental, R. Chronic, Including Teratogenic and Carcinogenic Effects of Trichothecenes: A Short Review. Vet. Res. Comm 1983, 7, 165–170. [Google Scholar]
- Khera, KS. Maternal Toxicity-A Possible Factor in Fetal Malformations in Mice. Teratology 1984, 29, 411–416. [Google Scholar]
- Schiefer, HB. Lethal Hemorrhages in Pregnant Mice Following One Oral Dose of T-2 Toxin. Arch. Belg. 1984, Suppl., 252–253. [Google Scholar]
- Hood, RD; Kuczuk, MH; Szczech, GM. Effects in Mice of Simultaneous Prenatal Exposure to Ochratoxin A and T-2 Toxin. Teratology 1978, 17, 25–30. [Google Scholar]
- Blakley, BR; Hancock, DS; Rousseaux, CG. Embryotoxic Effects of Prenatal T-2 Toxin Exposure in Mice. Can. J. Vet. Res 1987, 51, 399–403. [Google Scholar]
- Rousseaux, CG; Schiefer, HB. Maternal Toxicity, Embryolethality and Abnormal Fetal Development in CD-1 Mice Following One Oral Dose of T-2 Toxin. J. Appl. Toxicol 1987, 7, 281–288. [Google Scholar]
- Holladay, SD; Blaylock, BL; Comment, CE; Heindel, JJ; Luster, MI. Fetal Thymic Atrophy after Exposure to T-2 Toxin: Selectivity for Lymphoid Progenitor Cells. Toxicol. Appl. Pharmacol 1993, 121, 8–14. [Google Scholar]
- Holladay, SD; Smith, BJ; Luster, MIB. Lymphocyte Precursor Cells Represent Sensitive Targets of T2 Mycotoxin Exposure. Toxicol. Appl. Pharmacol 1995, 131, 309–315. [Google Scholar]
- Ishigami, N; Shinozuka, J; Katayama, K; Uetsuka, K; Nakayama, H; Doi, K. Apoptosis in the Developing Mouse Embryos from T-2 Toxin-inoculted Dams. Histol. Histopathol 1999, 14, 729–733. [Google Scholar]
- Ishigami, N; Shinozuka, J; Katayama, K; Nakayama, H; Doi, K. Apoptosis in Mouse Fetuses from Dams Exposed to T-2 Toxin at Different Days of Gestation. Exp. Toxicol. Pathol 2001, 52, 493–501. [Google Scholar]
- Sehata, S; Teranishi, M; Atsumi, F; Uetsuka, K; Nakayama, H; Doi, K. T-2 Toxin-induced Morphological Changes in Pregnant Rats. J. Toxicol. Pathol 2003, 16, 59–65. [Google Scholar]
- Sehata, S; Teranishi, M; Yamoto, T; Matsunuma, N; Doi, K. T-2 Toxin-Induced Toxicity in Pregnant Rats-Histopathology and Gene Expression Profiles-. In New Horizon of Mycotoxicology for Assuring Food Safety (Proceedings of ISMYCO Kagawa ’03); Yoshizawa, T, Ed.; Japanese Association of Mycotoxicology: Tokyo, 2004; pp. 33–39. [Google Scholar]
- Shinozuka, J; Tsutsui, S; Ishigami, N; Ueno-yamanouchi, A; Nakayama, H; Doi, K. Development of Apoptosis and Changes in Apoptosis-related Genes Expression in the Mouse Thymus Following T-2 Toxin-Inoculation. J. Toxicol. Pathol 1999, 12, 77–81. [Google Scholar]
- Shinozuka, J; Suzuki, H; Tsutsui, S; Nakayama, H; Doi, K. T-2 Toxin-induced Apoptosis and C-Fos mRNA Expression in ConA-Stimulated Mouse Thymocyte Primary Culture. J. Toxicol. Pathol 2001, 14, 247–251. [Google Scholar]
- Holme, JA; Morrison, E; Samuelsen, JT; Wiger, R; Lag, M; Schwarze, PE; Bernhoft, A; Refsnes, M. Mechanisms Involved in the Induction of Apoptosis by T-2 and HT-2 Toxins in HL-60 Human Promyelocytic Leukemia Cells. Cell. Biol. Toxicol 2003, 19, 53–68. [Google Scholar]
- Murshedul, AM; Nagase, M; Yoshizawa, T; Sakato, N. Thymocyte Apoptosis by T-2 Toxin in Vivo in Mice is Independent of Fas/Fas Ligand System. Biosci. Biotechnol. Biochem 2000, 64, 210–213. [Google Scholar]
- Rousseaux, CG; Nicholson, S; Schiefer, HB. Fatal Placental Hemorrhage in Pregnant CD-1 Mice Following One Oral Dose of T-2 Toxin. Can. J. Comp. Med 1985, 4, 95–98. [Google Scholar]
- Haynes, AW. Mycotoxin Teratogenicity. In Toxins: Animal, Plant and Microbial; Rosenberg, P, Ed.; Pergamon Press: New York, 1978; pp. 739–759. [Google Scholar]
- Gentry, PA; Cooper, ML. Effect of Fusarium T-2 Toxin on Hemorrhagical and Biochemical Parameters in the Rabbit. Can. J. Comp. Med 1981, 45, 400–405. [Google Scholar]
- Yarom, R; More, R; Eldor, A; Yagen, B. The Effect of T-2 Toxin on Human Platelets. Toxicol. Appl. Pharmacol 1984, 73, 210–217. [Google Scholar]
- Rousseaux, CG; Schiefer, HB; Hancock, DS. Reproductive and Teratological Effects of Continuous Low Level Dietary T-2 Toxin in Female CD-1 Mice for Two Generations. J. Appl. Toxicol 1986, 6, 179–184. [Google Scholar]
- Husmann, LA; Shimonkevitz, RP; Crispe, IN; Bevan, MJ. Thymocyte Subpopulation during Early Fetal Development in the BALB/c Mouse. J. Immunol 1988, 141, 736–740. [Google Scholar]
- Penit, C; Vaddeur, F. Cell Proliferation and Differentiation in Fetal and Early Postnatal Mouse Thymus. J. Immun 1989, 142, 3369–3377. [Google Scholar]
- Nagata, T; Suzuki, H; Ishigama, N; Shinozuka, J; Uetsuka, K; Nakayama, H; Doi, K. Development of Apoptosis and Changes in Lymphocyte Subsets in Thymus, Mesenteric Lymph Nodes and Peyer’s Patches of Mice Orally Inoculated with T-2 Toxin. Exp. Toxicol. Pathol 2001, 53, 309–315. [Google Scholar]
- Roberts, DW; Chapman, JR. Concepts Essential to the Assessment of Toxicity to the Developing Immune System. In Developmental Toxicology; Kimmel, CA, Buelke-Sam, J, Eds.; Raven Press: New York, 1981; pp. 167–189. [Google Scholar]
- Quiroga, MA; Risso, MA; Perfumo, CJ; Idiart, JR; Ohtsuka, R; Doi, K. Sequence of and Regional Difference in Apoptotic Index in the Mouse Gastrointestinal Mucous Epithelia after T-2 Toxin Inoculation. J. Toxicol. Pathol 2000, 13, 193–196. [Google Scholar]
- Fu, YT; Lin, WG; BaoCheng, Z; Quan, G. The Effect of T-2 Toxin on IL-1β and IL-6 Secretion in Human Fetal Chondrocytes. Int. Orthop 2001, 25, 199–201. [Google Scholar]
- Chen, J; Chu, Y; Cao, J; Yang, Z; Guo, X; Wang, Z. T-2 Toxin Induces Apoptosis, and Selenium Partly Blocks T-2 toxin-Induced Apoptosis in Chndrocytes through Modulation of the Bax/Bcl-2 Ratio. Food Chem. Toxicol 2006, 44, 567–573. [Google Scholar]
- Kniesel, U; Risua, W; Wolburg, H. Development of Blood-Brain Barrier Tight Junctions in the Rat Cortex. Brain Res. Dev. Brain Res 1996, 23, 229–240. [Google Scholar]
- Sehata, S; Kiyosawa, N; Makino, T; Atsumi, F; Ito, K; Yamoto, T; Teranishi, M; Baba, Y; Uetsuka, K; Nakayama, H; Doi, K. Morphological and Microarray Analysis of T-2 Toxin-induced Rat Fetal Brain Lesion. Food Chem. Toxicol 2004, 4, 1727–1736. [Google Scholar]
- Annunziato, L; Amoroso, S; Pannaccione, A; Cataldi, M; Pignataro, G; D’Alessio, A; Sirabella, R; Secondo, A; Sibaud, L; Di Renzo, GF. Apoptosis Induced in Neuronal Cells by Oxidative Stress: Role Played by Caspases and Intracellular Calcium Ions. Toxicol. Lett 2003, 139, 125–133. [Google Scholar]
- Troy, CM; Shelanski, ML. Caspase–2 Redux. Cell. Death Different 2003, 10, 101–107. [Google Scholar]
- Huang, P; Akagawa, K; Yokoyama, Y; Nohara, K; Kano, K; Morimoto, K. T-2 Toxin Initially Activates Caspase–2 and Induces Apoptosis in U937 Cells. Toxicol. Lett 2007, 170, 1–10. [Google Scholar]
- Katayama, K; Ueno, M; Yamauchi, H; Nagata, T; Nakayama, H; Doi, K. Ethylnitrosourea Induces Neuronal Progenitor Cell Apoptosis after S-Phase Accumulation in a p53-dependent Manner. Neurobiol. Dis 2005, 18, 218–225. [Google Scholar]
- Nam, C; Yamauchi, H; Nakayama, H; Doi, K. Etoposide Induces Apoptosis and Cell Cycle Arrest of Neuroepithelial Cells in a P53-dependent Manner. Neurotoxicol. Teratol 2006, 28, 664–672. [Google Scholar]
- Ueno, M; Katayama, K; Yamauchi, H; Nakayama, H; Doi, K. Cell Cycle and Cell Death Regulation of Neural Progenitor Cells in the 5-Azacytidine (5AzC)-treated Developing Fetal Brain. Exp. Neurol 2006, 198, 154–166. [Google Scholar]
- Woo, GH; Bak, EJ; Nakayama, H; Doi, K. Molecular Mechanisms of Hydroxyurea (HU)-induced Apoptosis in the Mouse Fetal Brain. Neurotoxicol. Teratol 2006, 28, 125–134. [Google Scholar]
- Yamauchi, H; Katayama, K; Ueno, M; Uetsuka, K; Nakayama, H; Doi, K. Involvement of P53 in 1-β-D-Arabinofuranosylcytosine-induced Rat Fetal Brain Lesions. Neurotoxicol. Teratol 2004, 26, 57–586. [Google Scholar]
- Sehata, S; Kiyosawa, N; Sakuma, K; Ito, K; Yamoto, T; Teranishi, M; Uetsuka, K; Nakayama, H; Doi, K. Gene Expression Profiles in Pregnant Rats Treated with T-2 Toxin. Exp. Toxicol. Pathol 2004, 55, 357–366. [Google Scholar]
- Sehata, S; Kiyosawa, N; Atsumi, F; Ito, K; Yamoto, T; Teranishi, M; Uetsuka, K; Nakayama, H; Doi, K. Microarray Analysis of T-2 Toxin-induced Liver, Placenta and Fetal Liver Lesions in Pregnant Rats. Exp. Toxicol. Pathol 2005, 57, 15–28. [Google Scholar]
- El Golli, E; Hassen, W; Bouslimi, A; Bouaziz, C; Ladjimi, MM; Bacha, H. Induction of Hsp 70 in Vero Cells in Response to Mycotoxins Cytoprotection by Sub-Lethal Heat Shock and by Vitamin E. Toxicol. Lett 2006, 166, 122–130. [Google Scholar]
- Yang, G; Jarvis, BB; Chung, J; Pestka, JJ. Apoptosis Induction by the Satratoxins and Other Trichothecene Mycotoxins: Relationship to ERK, P38 MAPK, and SAPK/JNK Activation. Toxicol. Appl. Pharmacol 2000, 15. [Google Scholar]
- Jaeschke, H; Gores, GJ; Cederbaum, AI; Hinson, JA; Pessayre, D; Lemasters, JJ. Mechanisms of Hepatotoxicity. Toxicol. Sci 2002, 65, 166–176. [Google Scholar]
- Vila, B; Jaradat, ZW; Marquardt, RR; Frohlich, AA. Effect of T-2 Toxin on in Vivo Lipid Peroxidation and Vitamin E Status in Mice. Food Chem. Toxicol 2002, 40, 479–486. [Google Scholar]
- Nagase, M; Alam, MM; Tsushima, A; Yoshizawa, T; Sakato, N. Apoptosis Induction by T-2 Toxin: Activation of Caspase–9, Caspase– 3, and DFF- 40/CAD through Cytosolic Release of Cytochrome c in HL- 60 Cells. Biosci. Biotechnol. Biochem 2001, 65, 1741–1947. [Google Scholar]
- Yamauchi, H; Katayama, K; Ueno, M; He, XJ; Mikami, T; Uetsuka, K; Doi, K; Nakayama, H. Essential Role of p53 in Trophoblastic Apoptosis Induced in the Developing Rodent Placenta by Treatment with a DNA-Damaging Agent. Apoptosis 2007, 12, 1943–1754. [Google Scholar]



© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doi, K.; Ishigami, N.; Sehata, S. T-2 Toxin-induced Toxicity in Pregnant Mice and Rats. Int. J. Mol. Sci. 2008, 9, 2146-2158. https://doi.org/10.3390/ijms9112146
Doi K, Ishigami N, Sehata S. T-2 Toxin-induced Toxicity in Pregnant Mice and Rats. International Journal of Molecular Sciences. 2008; 9(11):2146-2158. https://doi.org/10.3390/ijms9112146
Chicago/Turabian StyleDoi, Kunio, Noriaki Ishigami, and Shinya Sehata. 2008. "T-2 Toxin-induced Toxicity in Pregnant Mice and Rats" International Journal of Molecular Sciences 9, no. 11: 2146-2158. https://doi.org/10.3390/ijms9112146
APA StyleDoi, K., Ishigami, N., & Sehata, S. (2008). T-2 Toxin-induced Toxicity in Pregnant Mice and Rats. International Journal of Molecular Sciences, 9(11), 2146-2158. https://doi.org/10.3390/ijms9112146