Synthesis, Physical Characterization, Antibacterial and Antifungal Activities of 2-((E)-1-(2-((E)-1-(2-Hydroxyphenyl)ethylideneamino) phenylamino) ethyl) phenol
Abstract
:Introduction

Results and Discussion:
Antibacterial and antifungal activity tests
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Zalp-Yaman, S. E.; Kasumov, V. T.; Ahmet, M. O. Polyhedron 2005, 24, 1821.
- Samal, S.; Mohapatra, N. K.; Acharya, S.; Dey, R. K. Reac. & Func. Polym. 1999, 42, 37.
- KolodZiej, A. F. Prog. Inorg. Chem. 1994, 41, 493.
- Bouwman, E.; Henderson, R. K.; Reedijk, J.; Veldman, N.; Spek, A. L. Inorg. Chim. Acta 1999, 278, 105.
- Meseguer, M.; Moreno-Man, M.; Vallribera, A. Tetrahedron Lett. 2000, 41, 4093.
- Karmakar, R.; Choudhury, C. R.; Bravic, G.; Sutter, J. P.; Mitra, S. Polyhedron 2004, 23, 949.
- Mazloum Ardakani, M.; Salavati-Niasari, M.; Jamshidpoor, M. Sensors and Actuators 2004, 101, 102.
- Samal, S.; Acharya, S.; Dey, R. K.; Ray, A. R. Talanta 2002, 57, 1075.
- Frisch, M.J.; et al. GAUSSIAN 03, Revision A.1. Frisch, M. J., et al., Eds.; Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B.; Æ Frisch. Exploring Chemistry with Electronic Structure Methods, 2nd editionGaussian INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69, July 2001. National Institute of Standards and Technology: Gaithersburg MD; p. 20899, (http://webbook.nist.gov).
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503.
- Jalbout, A.F.; Jiang, Z.-Y.; Quasri, A.; Jeghnou, H.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Vib. Spect. 2003, 33, 21.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM). 2004, 627, 1, (Invited Review).
- Growth was measured in vitro using a liquid-phase method according to NCCLS guidelines from the American Society of Microbiology for 24 hours using various concentrations of drugs. Dei Cas, E.; Dujardin, L.; Ribeiro Pinto, M. E.; Ajana, F.; Fruit, J.; Poulain, D.; Camus, D.; Vernes, A.; Francois, N. Mycoses 1991, 34, 167–172. [PubMed]
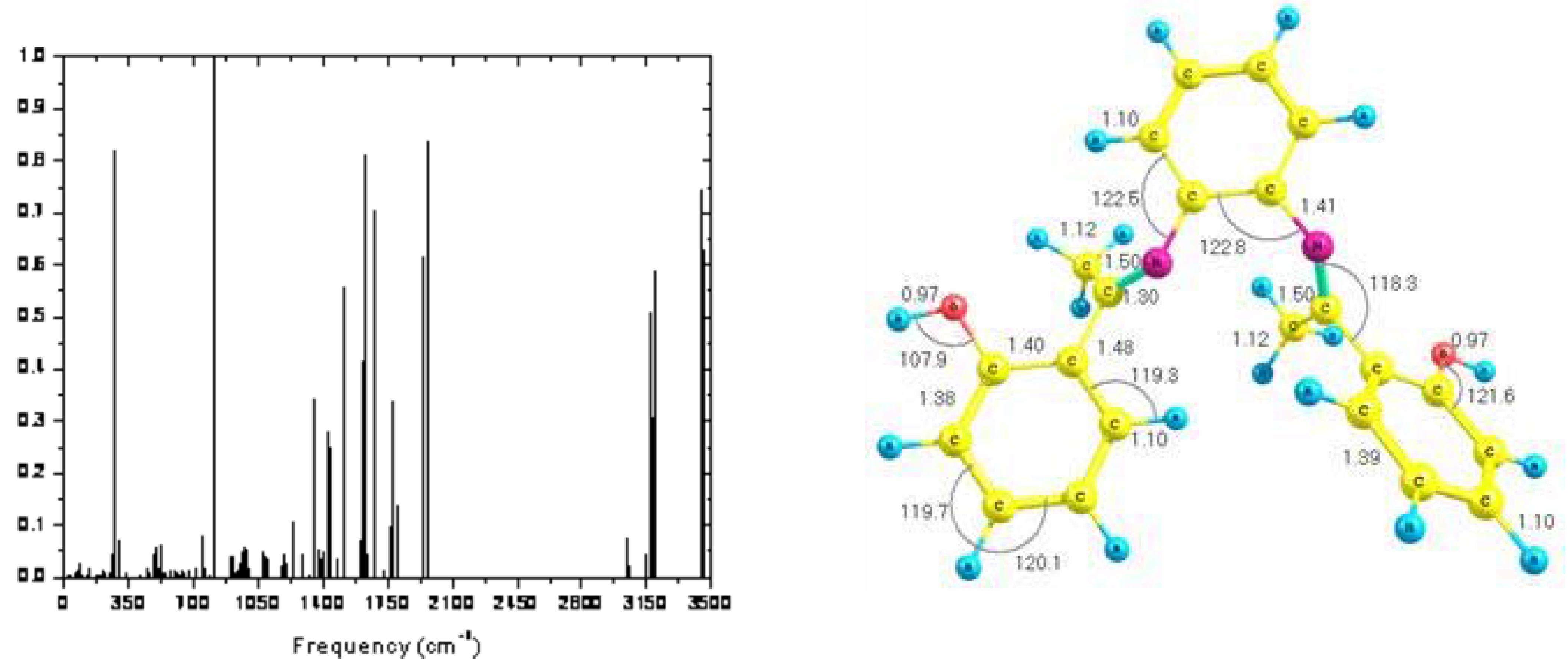
| Fitted Thermodynamic Equation (T/1000=t) | 100 K | 298.15 K | 1000 K | ||
| 3 | Cp | -43.40526+ 1651.94082*t -933.47093*t2 + 174.57639*t3 +0.49907*t-2 | 162.2 | 373.91 | 851.29 |
| S | 42.38515*ln(t) + 1150.64098*t + 29.96975*t2/2 -367.20075*t3/3 + 723.17337/(2*t2) + 162.84889 | 432.48 | 703.25 | 1444.07 | |
| ΔH | 133.5428*t +1417.88899*t2/2 -523.88367*t3/3 -52.55339*t4/4 +0.2264 /t -563.55851 | 10.32 | 63.16 | 526.8 | |
| Sample CIP | Antimicrobial activity (MIC), µg/mL | |||
| S. cerevisiae (ATCC 28383) | S. aureus (4.83) | C. albicans (1180-79) | E. Coli (54127) | |
| 3 | >50 | >50 | >50 | >50 |
© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Jalbout, A.F.; Brunel, J.M.; Loncle, C.; Rezaei, S.; Trzaskowski, B.T. Synthesis, Physical Characterization, Antibacterial and Antifungal Activities of 2-((E)-1-(2-((E)-1-(2-Hydroxyphenyl)ethylideneamino) phenylamino) ethyl) phenol. Molbank 2006, 2006, M489. https://doi.org/10.3390/M489
Jarrahpour AA, Jalbout AF, Brunel JM, Loncle C, Rezaei S, Trzaskowski BT. Synthesis, Physical Characterization, Antibacterial and Antifungal Activities of 2-((E)-1-(2-((E)-1-(2-Hydroxyphenyl)ethylideneamino) phenylamino) ethyl) phenol. Molbank. 2006; 2006(5):M489. https://doi.org/10.3390/M489
Chicago/Turabian StyleJarrahpour, A. A., A. F. Jalbout, J. M. Brunel, C. Loncle, S. Rezaei, and B. Trzaskowski Trzaskowski. 2006. "Synthesis, Physical Characterization, Antibacterial and Antifungal Activities of 2-((E)-1-(2-((E)-1-(2-Hydroxyphenyl)ethylideneamino) phenylamino) ethyl) phenol" Molbank 2006, no. 5: M489. https://doi.org/10.3390/M489




