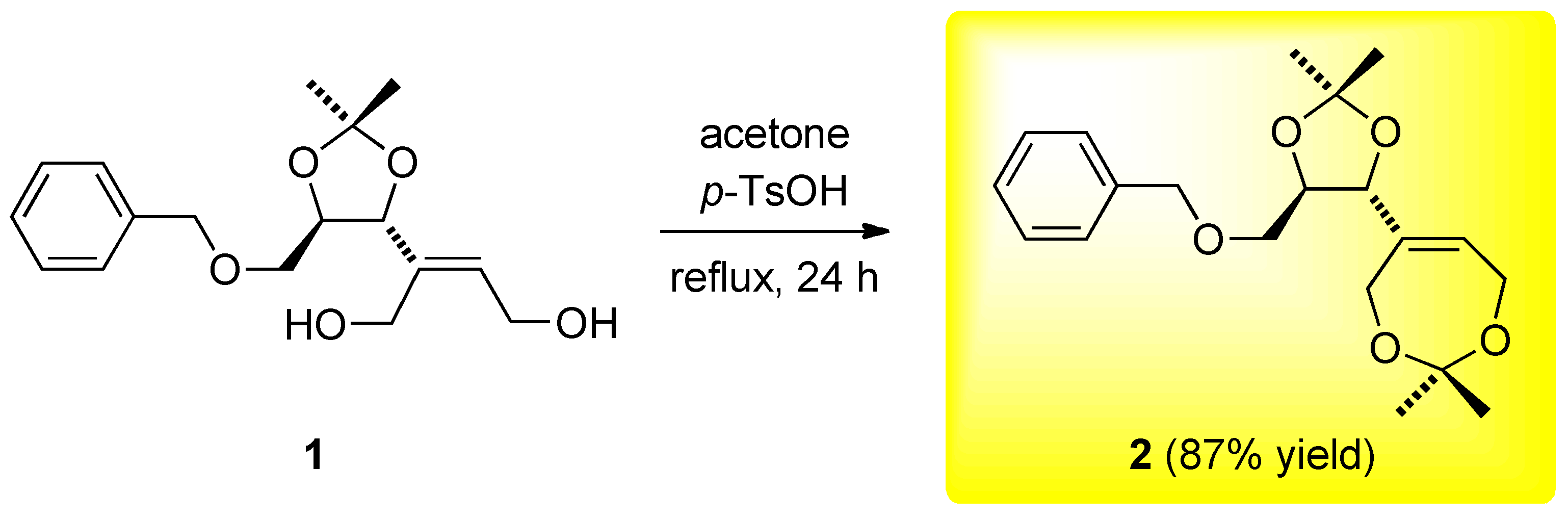
(–)-5-[(4R,5R)-5-(Benzyloxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-4,7-dihydro-1,3-dioxepine
Abstract
:Experimental
General
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References and Notes
- Philip, J.; Kocienski, P.J. Protective Groups, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2005. [Google Scholar]
- Maarse, H. Volatile Compounds in Foods and Beverages; Marcel Dekker: New York, NY, USA, 1991; p. 554. [Google Scholar]
- Stuart, A.; Garrie, S.A.; Wolf-Jürgensen, P. Effects of fluocinolone acetonide cream on the skin window record of inflammatory exudates. J. Invest. Dermatol. 1971, 57, 343–346. [Google Scholar]
- Carreras, C.R.; García, C.E.; Martín, V.S.; Tonn, C.E.; Díaz, D.D.; Ceñal, J.P. (E)-2-((4R,5R)-5-((Benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol. Molbank 2010, 2010, M676. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carreras, C.R.; García, C.E.; Martín, V.S.; Tonn, C.E.; Díaz, D.D.; Ceñal, J.P. (–)-5-[(4R,5R)-5-(Benzyloxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-4,7-dihydro-1,3-dioxepine. Molbank 2010, 2010, M680. https://doi.org/10.3390/M680
Carreras CR, García CE, Martín VS, Tonn CE, Díaz DD, Ceñal JP. (–)-5-[(4R,5R)-5-(Benzyloxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-4,7-dihydro-1,3-dioxepine. Molbank. 2010; 2010(2):M680. https://doi.org/10.3390/M680
Chicago/Turabian StyleCarreras, Carlos R., Celina E. García, Víctor S. Martín, Carlos E. Tonn, David Díaz Díaz, and Juan Pedro Ceñal. 2010. "(–)-5-[(4R,5R)-5-(Benzyloxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-4,7-dihydro-1,3-dioxepine" Molbank 2010, no. 2: M680. https://doi.org/10.3390/M680





