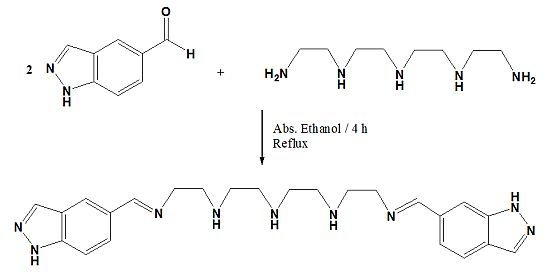
N1-((1H-Indazol-5-yl)methylene)-N2-(2-((2-((2-(((1H-indazol-6-yl)methylene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Sather, A.C.; Berryman, O.B.; Rebek, J., Jr. Synthesis of Fused Indazole Ring Systems and Application to Nigeglanine Hydrobromide. Org. Lett. 2012, 14, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ferreira, D.; Carvalho, P.; Avery, M.A.; Khan, I.A. Nigellidine-4-O-sulfite, the First Sulfated Indazole-Type Alkaloid from the Seeds of Nigella sativa. J. Nat. Prod. 2008, 71, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Pelletier, J.C.; Hodge, C.N. Tricyclic ureas: A new class of HIV-1 protease inhibitor. Bioorg. Med. Chem. Lett. 1998, 8, 3615–3620. [Google Scholar] [CrossRef]
- Patel, M.; Rodgers, J.D.; McHugh, R.J., Jr.; Johnson, B.L.; Cordova, B.C.; Klaba, R.M.; Bacheler, L.T.; Erickson-Viitanen, S.; Ko, S.S. Unsymmetrical cyclic ureas as HIV-1 protease inhibitors: Novel biaryl indazoles as P2/P2′ substituents. Bioorg. Med. Chem. Lett. 1999, 9, 3217–3220. [Google Scholar] [CrossRef]
- Sun, J.-H.; Teleha, C.A.; Yan, J.-S.; Rodgers, J.D.; Nugiel, D.A. Efficient Synthesis of 5-(Bromomethyl)- and 5-(Aminomethyl)-1-THP-Indazole. J. Org. Chem. 1997, 62, 5627–5629. [Google Scholar] [CrossRef]
- Mosti, L.; Menozzi, G.; Fossa, P.; Filippelli, W.; Gessi, S.; Rinaldi, B.; Falcone, G. Synthesis and preliminary biological evaluation of novel N-substituted 1-amino-3-[1-methyl(phenyl)-1H-indazol-4-yloxy]-propan-2-ols interesting as potential antiarrhythmic, local anaesthetic and analgesic agents. Arzneimittelforschung 2000, 50, 963–972. [Google Scholar] [PubMed]
- Jakupec, M.A.; Reisner, E.; Eichinger, A.; Pongratz, M.; Arion, V.B.; Galanski, M.; Hartinger, C.G.; Keppler, B.K. Redox-Active Antineoplastic Ruthenium Complexes with Indazole: Correlation of in Vitro Potency and Reduction Potential. J. Med. Chem. 2005, 48, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Showalter, H.D.H.; Angelo, M.M.; Berman, E.M.; Kanter, G.D.; Ortwine, D.F.; Ross-Kesten, S.G.; Sercel, A.D.; Turner, W.R.; Werbel, L.M.; Worth, D.F.; et al. Benzothiopyranoindazoles, a New Class of Chromophore Modified Anthracenedione Anticancer Agents. Synthesis and Activity against Murine Leukemias. J. Med. Chem. 1988, 31, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.B.; Cui, H.; Dowdell, S.E.; Gaitanopoulos, D.E.; Ivy, R.L.; Sehon, C.A.; Stavenger, R.A.; Wang, G.Z.; Viet, A.Q.; Xu, W.; et al. Development of Dihydropyridone Indazole Amides as Selective Rho-Kinase Inhibitors. J. Med. Chem. 2007, 50, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Daidone, G.; Maggio, B.; Schillaci, D.; Plescia, F. Synthesis and Antiproliferative Activity of Novel 3-(Indazol-3-yl)-quinazolin-4(3H)-one and 3-(Indazol-3-yl)-benzotriazin-4(3H)-one Derivatives. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 317–320. [Google Scholar] [CrossRef]
- Lodeiro, C.; Pina, F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord. Chem. Rev. 2009, 253, 1353–1385. [Google Scholar] [CrossRef]
- Lodeiro, C.; Capelo, J.L.; Mejuto, J.C.; Oliveira, E.; Santos, H.M.; Pedras, B.; Nuñez, C. Light and colour as analytical detection tools: A journey into the periodic table using polyamines to bio-inspired systems as chemosensors. Chem. Soc. Rev. 2010, 39, 2948–2976. [Google Scholar] [CrossRef] [PubMed]
- Bastista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Imidazo-benzo-15-crwon-5 ethers bearing arylthienyl and bithienyl moieitis as novel fluorescent chemosensor for Pd(II) and Cu(II). Tetrahedron 2011, 67, 7106–7113. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lodeiro, J.; Nuñez, C.; Carreira, R.; Santos, H.M.; López, C.S.; Mejuto, J.C.; Capelo, J.L.; Lodeiro, C. Novel versatile imine-enamine chemosensor based on 6-nitro-4-oxo-4H-chromene for ion detection in solution, solid and gas phase: Synthesis, emission, computational and MALDI-TOF-MS studies. Tetrahedron 2011, 67, 326–333. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Núñez, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Bértolo, E.; Capelo, J.L.; Lodeiro, C. N1-((1H-Indazol-5-yl)methylene)-N2-(2-((2-((2-(((1H-indazol-6-yl)methylene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine. Molbank 2012, 2012, M770. https://doi.org/10.3390/M770
Núñez C, Fernández-Lodeiro J, Fernández-Lodeiro A, Bértolo E, Capelo JL, Lodeiro C. N1-((1H-Indazol-5-yl)methylene)-N2-(2-((2-((2-(((1H-indazol-6-yl)methylene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine. Molbank. 2012; 2012(3):M770. https://doi.org/10.3390/M770
Chicago/Turabian StyleNúñez, Cristina, Javier Fernández-Lodeiro, Adrián Fernández-Lodeiro, Emília Bértolo, José Luis Capelo, and Carlos Lodeiro. 2012. "N1-((1H-Indazol-5-yl)methylene)-N2-(2-((2-((2-(((1H-indazol-6-yl)methylene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine" Molbank 2012, no. 3: M770. https://doi.org/10.3390/M770
APA StyleNúñez, C., Fernández-Lodeiro, J., Fernández-Lodeiro, A., Bértolo, E., Capelo, J. L., & Lodeiro, C. (2012). N1-((1H-Indazol-5-yl)methylene)-N2-(2-((2-((2-(((1H-indazol-6-yl)methylene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine. Molbank, 2012(3), M770. https://doi.org/10.3390/M770