(S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
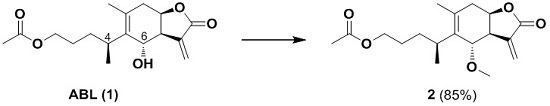
3.2. Synthesis of (S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate (or Named: 1-O-Acetyl-6-methoxybritannilactone)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, B.-N.; Bai, N.-S.; Lin, L.-Z.; Cordell, G. Sesquiterpene lactones from Inula britannica. Phytochemistry 1993, 34, 249–252. [Google Scholar]
- Fang, X.M.; Liu, B.; Liu, Y.B.; Wang, J.J.; Wen, J.K.; Li, B.H.; Han, M. Acetylbritannilactone suppresses growth via upregulation of kruppel-like transcription factor 4 expression in HT-29 colorectal cancer cells. Oncol. Rep. 2011, 26, 1181–1187. [Google Scholar] [PubMed]
- Ho, C.-T.; Rafi, M.; Dipaola, R.S.; Ghai, G.; Rosen, R.T.; Bai, N. Inducing Cell Apoptosis and Treating Cancer Using 1-O-Acetylbritannilactone or 1,6-O,O-Diacetylbritannilactone. U.S. Patent 6627623, 30 September 2003. [Google Scholar]
- Qi, J.-L.; Fu, Y.; Shi, X.-W.; Wu, Y.-B.; Wang, Y.-Z.; Zhang, D.-Q.; Shi, Q.-W. Sesquiterpene lactones and their anti-tumor activity from the flowers of Inula britannica. Lett. Drug Des. Discov. 2008, 5, 433–436. [Google Scholar] [CrossRef]
- Je, K.-H.; Han, A.-R.; Lee, H.-T.; Mar, W.; Seo, E.-K. The inhibitory principle of lipopolysaccharide-induced nitric oxide production from Inula britannica var. Chinensis. Arch. Pharm. Res. 2004, 27, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, M.; Wen, J.K. Acetylbritannilactone inhibits neointimal hyperplasia after balloon injury of rat artery by suppressing nuclear factor-kappa B activation. J. Pharmacol. Exp. Ther. 2008, 324, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Bai, N.-S.; Ho, C.-T.; Rosen, R.T.; White, E.; Perez, D.; DiPaola, R.S. A sesquiterpenelactone from Inula britannica induces anti-tumor effects dependent on Bcl-2 phosphorylation. Anticancer Res. 2005, 25, 313–318. [Google Scholar] [PubMed]
- Han, M.; Wen, J.K.; Zheng, B.; Zhang, D.Q. Acetylbritannilatone suppresses NO and PGE2 synthesis in Raw 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci. 2004, 75, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chiou, Y.S.; Cheng, A.C.; Bai, N.; Lo, C.Y.; Tan, D.; Ho, C.T. Involvement of mapk, Bcl-2 family, cytochrome c, and caspases in induction of apoptosis by 1,6-O,O-diacetylbritannilactone in human leukemia cells. Mol. Nutr. Food Res. 2007, 51, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Wen, J.K.; Wu, Y.B.; Zhang, J.; Zheng, B.; Zhang, D.Q.; Han, M. 1,6-O,O-diacetylbritannilactones inhibits IkappaB kinase beta-dependent NF-kappaB activation. Phytomedicine 2009, 16, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Tang, J.-J.; Zhang, C.-C.; Tian, J.-M.; Guo, J.-T.; Zhang, Q.; Li, H.; Gao, J.-M. Semisynthesis and in vitro cytotoxic evaluation of new analogues of 1-O-acetylbritannilactone, a sesquiterpene from Inula britannica. Eur. J. Med. Chem. 2014, 80, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Yan, W.; Zhang, L.; Bai, N.; Ho, C.T. Studies on 1-O-acetylbritannilactone and its derivative, (2-O-butyloxime-3-phenyl)-propionyl-1-O-acetylbritannilactone ester. Bioorg. Med. Chem. Lett. 2004, 14, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Yan, W.; Zhang, L.; Bai, N.; Ho, C.T. Design, synthesis, and anti-tumor activity of (2-O-alkyloxime-3-phenyl)-propionyl-1-O-acetylbritannilactone esters. Bioorg. Med. Chem. 2005, 13, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, L.; Fu, Y.; Yuan, Z.; Wang, Z.; Zhang, L. Pharmaceutical Application of Derivant of Benzofuran-Ketone. CN1445221A, 1 October 2003. [Google Scholar]
- Tang, J.J.; Dong, S.; Han, Y.Y.; Lei, M.; Gao, J.M. Synthesis of 1-O-acetylbritannilactone analogues from Inula britannica and in vitro evaluation of their anticancer potential. Med. Chem. Comm. 2014, 5, 1584–1589. [Google Scholar] [CrossRef]

| No. | IC50 1 (μM) | ||
|---|---|---|---|
| HCT116 | SGC-7901 | HeLa | |
| ABL (1) | 36.1 ± 3.1 | 42.5 ± 6.1 | 32.6 ± 2.5 |
| 2 | 35.7 ± 5.2 | 37.5 ± 3.8 | 38.6 ± 2.9 |
| VP-16 | 2.13 ± 0.23 | 6.56 ± 0.68 | 2.97 ± 0.25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Gao, J.-M.; Tang, J.-J. (S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate. Molbank 2016, 2016, M890. https://doi.org/10.3390/M890
Guo X, Gao J-M, Tang J-J. (S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate. Molbank. 2016; 2016(1):M890. https://doi.org/10.3390/M890
Chicago/Turabian StyleGuo, Xin, Jin-Ming Gao, and Jiang-Jiang Tang. 2016. "(S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate" Molbank 2016, no. 1: M890. https://doi.org/10.3390/M890
APA StyleGuo, X., Gao, J.-M., & Tang, J.-J. (2016). (S)-4-[(3aR,4S,7aR)-4-Methoxy-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-5-yl]pentyl Acetate. Molbank, 2016(1), M890. https://doi.org/10.3390/M890