5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole
Abstract
:1. Introduction
2. Results
3. Discussion
4. Experimental Section
4.1. General Informations
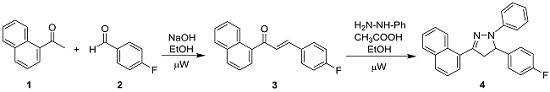
4.2. Synthesis of 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole (4)
5. Conclusions
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palaska, E.; Aytemir, M.; Uzbay, I.T.; Erol, D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001, 36, 539–543. [Google Scholar] [CrossRef]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.D.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of novel analogs of 2-pyrazoline obtained from[(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M.; Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Mohamad, Y. Synthesis, studies and in vitro antibacterial activity of some 5-(thiophene-2-yl)-phenyl pyrazoline derivatives. J. Saudi Chem. Soc. 2014, 18, 411–417. [Google Scholar] [CrossRef]
- Viveka, S.; Dinesha; Shama, P.; Nagaraja, G.K.; Ballav, S.; Kerkar, S. Design and synthesis of some new pyrazolyl-pyrazolines as potential anti-inflammatory, analgesic and antibacterial agents. Eur. J. Med. Chem. 2015, 101, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Bansal, E.; Srivastava, V.K.; Kumar, A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substitute daryl-3-(β-aminonaphthyl)-2-pyrazolines and β-(substituted aminoethyl) amidonaphthalenes. Eur. J. Med. Chem. 2001, 36, 81–92. [Google Scholar] [CrossRef]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Dhulap, A.; Ali, Y.; Nazreen, S.; Haider, S. Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents. Bioorg. Med. Chem. 2014, 22, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, L.; Wei, Z.; Zhu, J.; Peng, F.; Shao, M.; Lei, L.; He, L.; Tang, M.; He, L.; et al. Synthesis and biological evaluation of novel pyrazoline derivatives as potent anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2015, 25, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, J.; Wang, M.; Zhou, Y.; Chen, X. Synthesis and fluorescent property of pyrazoline derivatives. Chin. J. Chem. 2012, 30, 1345–1350. [Google Scholar] [CrossRef]
- Ramkumar, V.; Kannan, P. Thiophene and furan containing pyrazoline luminescent materials for optoelectronics. J. Lumin. 2016, 169 Part A, 204–215. [Google Scholar] [CrossRef]
- Ramkumar, V.; Kannan, P. Highly fluorescent semiconducting pyrazoline materials for optoelectronics. Opt. Mater. 2015, 46, 605–613. [Google Scholar] [CrossRef]
- Singh, P.; Negi, J.S.; Pant, G.J.; Rawat, M.S.M.; Budakoti, A. Synthesis and Characterization of a Novel 2-Pyrazoline. Molbank 2009, 2009, M614. [Google Scholar] [CrossRef]
- Razzaq, T.; Kappe, C.O. On the energy efficiency of microwave-assisted organic reactions. ChemSusChem 2008, 1, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vaismaa, M. Development of benign synthesis of some terminal α-hydroxy ketones and aldehydes. Dissertation; Department of Chemistry, Faculty of Science, University of Oulu: Oulu, Finland, 21 August 2009. Available online: http://urn.fi/urn:isbn:9789514291753 (accessed on 31 December 2015).
- Seijas, J.A.; Va’zquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem. 2005, 70, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Vijayaramalingam, K.; Chandrasekaran, S.; Nagarajan, S. Synthesis and antibacterial activity of 4-(1-naphthyl)-6-arylpyrimidin-2-(1H)-ones. Pharm. Chem. J. 2007, 41, 253–255. [Google Scholar] [CrossRef]
- Espinosa, J.F. Determination of magnitudes and relative signs of 1H-19F coupling constants through 1D- and 2D-TOCSY experiments. J. Org. Chem. 2013, 78, 12844–12847. [Google Scholar]
- Richards, S.A.; Hollerton, J.C. Essential Practical NMR for Organic Chemistry; John Wiley and Sons, Ltd: Singapore, Singapore, 2011; pp. 84–86. [Google Scholar]


| Position | δC (ppm), Type (J in Hz) | δH (ppm), (J in Hz) | HMBC |
|---|---|---|---|
| 3 | 147.3, C | - | - |
| 4 | 46.1, CH2 | H4a: 3.36, dd (17.0, 7.0) | 3, 5, 1′ |
| H4b: 4.07, dd (17.0, 12.5) | |||
| 5 | 62.6, CH | 5.30, dd (12.5, 7.0) | 3, 4, 1′, 2′ |
| 1′ | 138.2, d, C (3.8) | - | - |
| 2′ | 127.6, d, CH (7.5) | 7.36, dd (8.5, 5.5) | 5, 4′, 6′ |
| 3′ | 116.1, d, CH (22.5) | 7.05, t (8.5) | 1′, 4′, 5′ |
| 4′ | 162.1, d, C (244.0) | - | - |
| 5′ | 116.1, d, CH (22.5) | 7.05, t (8.5) | 1′, 3′, 4′ |
| 6′ | 127.6, d, CH (7.5) | 7.36, dd (8.5, 5.5) | 5, 2′, 4′ |
| 1′′ | 128.9, C | - | - |
| 2′′ | 126.7, CH | 7.47, d (7.0) | 4′′, 9′′ |
| 3′′ | 124.9, CH | 7.44, t (7.0) | 1′′, 10′′ |
| 4′′ | 129.7, CH | 7.84, dd (7.0, 1.0) | 2′′, 5′′, 9′′ |
| 5′′ | 128.6, CH | 7.91, d (8.5) | 4′′, 7′′, 9′′ |
| 6′′ | 126.2, CH | 7.59, dt (8.0, 1.0) | 8′′, 10′′ |
| 7′′ | 127.4, CH | 7.71, dt (8.0, 1.0) | 5′′, 9′′ |
| 8′′ | 127.3, CH | 9.56, d (8.5) | 1′′, 6′′, 10′′ |
| 9′′ | 130.6, C | - | - |
| 10′′ | 134.2, C | - | - |
| 1′′′ | 144.6, C | - | - |
| 2′′′ | 113.5, CH | 7.14, d (7.5) | 3′′′, 4′′′, 6′′′ |
| 3′′′ | 129.1, CH | 7.26, t (7.5) | 1′′′, 5′′′ |
| 4′′′ | 119.4, CH | 6.85, t (7.5) | 2′′′, 3′′′, 5′′′ |
| 5′′′ | 129.1, CH | 7.26, t (7.5) | 1′′′, 3′′′ |
| 6′′′ | 113.5, CH | 7.14, d (7.5) | 2′′′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasril, J.; Zamri, A.; Ikhtiarudin, I.; Teruna, H.Y. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole. Molbank 2016, 2016, M891. https://doi.org/10.3390/M891
Jasril J, Zamri A, Ikhtiarudin I, Teruna HY. 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole. Molbank. 2016; 2016(2):M891. https://doi.org/10.3390/M891
Chicago/Turabian StyleJasril, Jasril, Adel Zamri, Ihsan Ikhtiarudin, and Hilwan Y. Teruna. 2016. "5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole" Molbank 2016, no. 2: M891. https://doi.org/10.3390/M891
APA StyleJasril, J., Zamri, A., Ikhtiarudin, I., & Teruna, H. Y. (2016). 5-(4-Fluorophenyl)-3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazole. Molbank, 2016(2), M891. https://doi.org/10.3390/M891