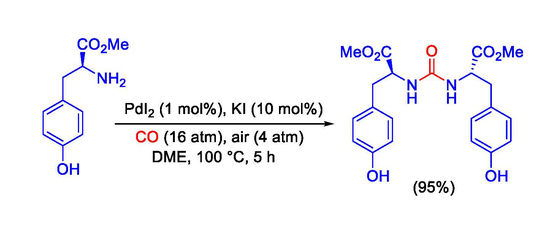
Dimethyl 2,2′-[Carbonylbis(azanediyl)](2S,2′S)-bis[3-(4-hydroxyphenyl)propanoate]
Abstract
:1. Introduction
2. Results
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, H.-Q.; Lv, P.-C.; Yan, T.; Zhu, H.-L. Urea derivatives as anticancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 471–480. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Kondekar, N.B.; Sadani, A.A.; Chern, J.-W. Ureas: applications in drug design. Curr. Med. Chem. 2017, 24, 622–651. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M. Urea derivatives as low-molecular-weight gelators. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 33–48. [Google Scholar] [CrossRef]
- Volz, N.; Clayden, J. The urea renaissance. Angew. Chem. Int. Ed. 2011, 50, 12148–12155. [Google Scholar] [CrossRef] [PubMed]
- Beller, M. (Ed.) Catalytic Carbonylation Reactions; Springer: Berlin, Germany, 2006; ISBN 978-3-540-33003-5. [Google Scholar]
- Diaz, D.J.; Darko, A.K.; McElwee-White, L. Transition metal-catalyzed oxidative carbonylation of amines to ureas. Eur. J. Org. Chem. 2007, 2007, 4453–4465. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Costa, M. A novel and efficient method for the Pd-catalysed oxidative carbonylation of amines to symmetrically and unsymmetrically substituted ureas. Chem. Commun. 2003, 2003, 467–487. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient synthesis of ureas by direct palladium-catalyzed oxidative carbonylation of amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-catalyzed synthesis of symmetrical urea derivatives by oxidative carbonylation of primary amines in carbon dioxide medium. J. Catal. 2011, 282, 120–127. [Google Scholar] [CrossRef]
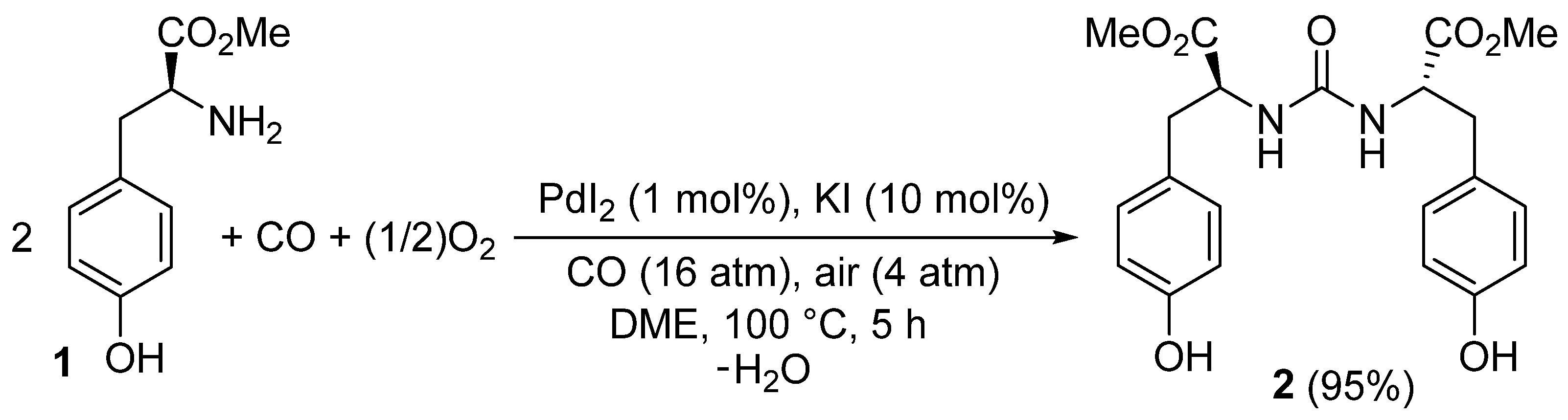

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.; Gioia, K.F.; Piccionello, A.P.; Della Ca’, N.; Carfagna, C.; Gabriele, B. Dimethyl 2,2′-[Carbonylbis(azanediyl)](2S,2′S)-bis[3-(4-hydroxyphenyl)propanoate]. Molbank 2018, 2018, M983. https://doi.org/10.3390/M983
Mancuso R, Gioia KF, Piccionello AP, Della Ca’ N, Carfagna C, Gabriele B. Dimethyl 2,2′-[Carbonylbis(azanediyl)](2S,2′S)-bis[3-(4-hydroxyphenyl)propanoate]. Molbank. 2018; 2018(1):M983. https://doi.org/10.3390/M983
Chicago/Turabian StyleMancuso, Raffaella, Katia F. Gioia, Antonio Palumbo Piccionello, Nicola Della Ca’, Carla Carfagna, and Bartolo Gabriele. 2018. "Dimethyl 2,2′-[Carbonylbis(azanediyl)](2S,2′S)-bis[3-(4-hydroxyphenyl)propanoate]" Molbank 2018, no. 1: M983. https://doi.org/10.3390/M983