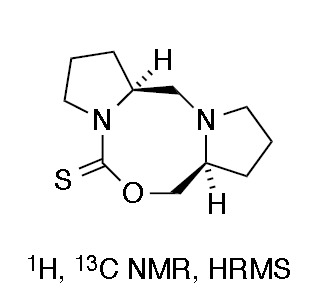
Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione
Abstract
:1. Introduction
2. Results
3. Experimental Section
Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione (2)
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Aitken, R.A.; Ali, K.; Mesher, S.T.E. Kinetic resolution of secondary alcohols using proline-derived bicyclic iminium salts. Tetrahedron Lett. 1997, 38, 4179–4182. [Google Scholar] [CrossRef]
- Mahy, W.; Plucinski, P.; Jover, J.; Frost, C.G. Ruthenium-catalyzed O- to S-alkyl migration: A pseudoreversible Barton-McCombie pathway. Angew. Chem. Int. Ed. 2015, 54, 10944–10948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabb, T.A.; Jackson, G.C. Proton magnetic resonance studies of compounds with bridgehead nitrogen. XXVIII—Stereochemical studies with Perhydropyrido[1,2-c][1,6,3]dioxazocines and 2-alkylperhydropyrido[1,2-c][1,3,6]oxadiazocines. Org. Magn. Reson. 1975, 7, 488–495. [Google Scholar] [CrossRef]
- Nishiyama, T.; Fujiwara, T.; Miyazaki, S.; Yamada, F. Cis and Trans 4,8-dimethyl-6-phenyl-5,6,7,8-tetrahydro-4H-1,3,6-oxadiazocin-2-ones: Conformational studies and configurational assignments by NMR. J. Heterocycl. Chem. 1992, 29, 1529–1533. [Google Scholar] [CrossRef]
- Pulacchini, S.; Watkinson, M. An Efficient Route to Symmetrically and Unsymmetrically Substituted Azamacrocyclic Ligands. Eur. J. Org. Chem. 2001, 4233–4238. [Google Scholar] [CrossRef]
- Fontanella, L.; Occelli, E. Farmaci potenzialmente attivi sul sistema nervoso centrale derivati del 5-oxa-3,10-diazabiciclo[5.2.1]decano e del 5-oxa-3,10-diazabiciclo[5.2.1]decan-4-one. Farmaco Ed. Scient. 1971, 26, 685–709. (In Italian) [Google Scholar]
- Achour, R.; Essassi, E.M.; Zniber, R.; Desvergne, J.-P.; Bouas-Laurent, H. Réactivité photochimique en solution de 1,5-diazodiazepinones. Bull. Soc. Chim. Belg. 1993, 102, 479–491. (In French) [Google Scholar] [CrossRef]
- Hornyak, G.; Lempert, K. Benzoxadiazocines, benzothiadiazocines and benzotriazocines I: General considerations and the synthesis of two 3,1,6-benzoxadiazocines. Tetrahedron 1983, 39, 479–481. [Google Scholar] [CrossRef]
- Daniel, J.; Dhar, D.N. A facile synthesis of triazocinones and oxadiazocinones. Synth. Commun. 1993, 23, 2151–2157. [Google Scholar] [CrossRef]
- Griengl, H.; Hayden, W.; Plessing, A. C(6)-C(7)-cyclised purines. J. Heterocycl. Chem. 1984, 21, 333–336. [Google Scholar] [CrossRef]
- Musonda, C.C.; Edlin, C.D.; Boyle, G.A. Nitroimidazooxadiazocine Compounds. P.C.T. International Patent Application WO 2013 072903 A1, 23 May 2013. [Google Scholar]
- Sonopo, M.S.; Venter, K.; Boyle, G.; Winks, S.; Marjanovic-Painter, B.; Zeevaart, J.R. Carbon-14 radiolabeling and in vivo biodistribution of a potential anti-TB compound. J. Labelled Compd. Radiopharm. 2015, 58, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Diness, F.; Beyer, J.; Meldal, M. Solid-Phase Synthesis of Terahydro-β-carbolines and Tetrahydroisoquinolines by Stereoselective Intramolecular N-Carbamyliminium Pictet-Spengler Reactions. Chem. Eur. J. 2006, 12, 8056–8066. [Google Scholar] [CrossRef] [PubMed]
- Horikiri, T.; Yoshihiko, K. Gel Electrolyte, Cell and Electrochemical Element. European Patent EP 0996029 A2, 26 April 2000. [Google Scholar]
- Guichard, G.; Lena, G.; Muller, P.; Rognan, D.; Boilard, E.; Lambeau, G. Compositions and Methods for the Inhibition of Phospholipase A2. U.S. Patent 2008 031951 A1, 7 February 2008. [Google Scholar]
- Guichard, G.; Lena, G.; Muller, P.; Rognan, D.; Lambeau, G. Compositions and Methods for the Inhibition of Phospholipase A2. P.C.T. International Patent Application WO 2007 074169 A2, 5 July 2007. [Google Scholar]
- Guichard, G.; Lena, G.; Lallemand, E.; Renia, L. Compositions and Methods for the Treatment of Disease. P.C.T. International Patent Application WO 2007 074170 A2, 5 July 2007. [Google Scholar]
- Guichard, G.; Lena, G.; Lallemand, E.; Renia, L. Aza heterocyclics for the Treatment of Malaria or AIDS. P.C.T. International Patent Application WO 2007 074171 A1, 5 July 2007. [Google Scholar]
- Bao, R.; Huang, W.; Zhen, Y.; He, B. One-pot synthesis of 4-substituted 2-thio-5-oxo-1,3,6-thiadiazaoctane. Synth. Commun. 1990, 20, 2675–2682. [Google Scholar] [CrossRef]
- Enders, D.; Eichenauer, H. Asymmetrische Synthesen via metallierte chirale hydrazone. Enantioselektive Alkylierung von cyclischen Ketonen und Aldehyden. Eur. J. Inorg. Chem. 1979, 112, 2933–2960. [Google Scholar] [CrossRef]




| δC | mult. (DEPT) | δH (HSQC) | Coupled H (COSY) | Assignment |
|---|---|---|---|---|
| 190.5 | C | — | 5-C=S | |
| 78.9 | CH2 | 4.08, 4.16 | 7-CH2O | |
| 62.6 | CH | 3.10 | 4.08 | 7a-CHN |
| 60.0 | CH | 4.55 | 2.53, 2.08 | 12a-CHN |
| 58.3 | CH2 | 2.53 (2H) | 12-CH2N | |
| 56.7 | CH2 | 3.10, 2.56 | 10-CH2N | |
| 50.1 | CH2 | 3.80, 3.66 | 2.08 | 3-CH2N |
| 28.6 | CH2 | † | 1-CH2 * | |
| 27.2 | CH2 | † | 8-CH2 * | |
| 24.4 | CH2 | † | 2-CH2 * | |
| 22.4 | CH2 | † | 9-CH2 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Ali, K. Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione. Molbank 2018, 2018, M993. https://doi.org/10.3390/M993
Aitken RA, Ali K. Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione. Molbank. 2018; 2018(2):M993. https://doi.org/10.3390/M993
Chicago/Turabian StyleAitken, R. Alan, and Karamat Ali. 2018. "Octahydro-1H,5H,7H-dipyrrolo[1,2-c:1′,2′-f][1,3,6]oxadiazocine-5-thione" Molbank 2018, no. 2: M993. https://doi.org/10.3390/M993