Abstract
The Biginelli reaction is a highly versatile reaction that leads to dihydropyrimidinones/thiones. This scaffold is reported as being a privileged structure due to its ability to interact with biological targets. Synthesis of ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate was achieved through the Biginelli reaction using a functionalized thiourea. In silico studies demonstrated that the compound title showed good potential for interacting with ecto-5’-nucleotidase, which has been considered as a target in designs for anti-cancer drugs.
1. Introduction
The Biginelli reaction involves the acid-catalyzed multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-ones(thiones) (DHPMs) [1]. Thus, this reaction is a powerful tool for the fast and easy generation of libraries with great structural diversity [2]. DHPMs are an example of privileged scaffolds due to their interaction with numerous biological targets [3].
It has been recently identified that N1 substituted Biginelli compounds were able to interact with Eg5, a protein involved in chromosome separation during the cell cycle [4]. Another target for this heterocycle class is the enzyme ecto-5′-nucleotidase (5′-NT or CD73), a protein involved in the hydrolysis of adenosine monophosphate (AMP) to adenosine, a molecule that increases cancer growth through immune system suppression [5]. DHPM LaSOM 63 was identified as a prototype for the development of an ecto-5′-nucleotidase inhibitor. LaSOM 63 was active against glioma cells and inhibited the phosphate liberation from cells treated with AMP [6]. In this context, in continuation with our studies focused on exploring the possibility of finding a DHPM ecto-5′-nucleotidase inhibitor, a new N1-aryl substituted DHPM was designed and synthesized.
2. Results and Discussion
A virtual library containing 528 compounds was constructed from dihydropyrimidinone scaffolds. For this purpose, 22 thioureas and 24 aldehydes were included in the SMILIB program [7]. SMILIB is able to construct very large combinatorial compound libraries in the SMILES format.
Following this, 87 compounds were selected for their physicochemical and pharmacokinetic properties. The filter was based on data obtained by the SwissADME server [8] using the Lipinski rule [9], and the ability to permeate the brain blood-brain barrier. These 35 selected compounds were submitted into two virtual screening routines: ligand-based, using the SHApe-FeaTure Similarity (SHAFTS) program [10] (ShapeSim = 0.625), and structure-based, using the Autodock Vina program [11] (score = −6.41 kJ/mol). Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (6) LaSOM 282 was selected by consensus rank-by-rank between compounds more similar to adenosine and the compound with greater affinity to the ecto-5′-nucleotidase receptor.
The title compound LaSOM 282 (6) was synthesized by the multicomponent Biginelli reaction promoted by trimethylsilane chloride (TMSCl) from condensation of ethyl acetoacetate (4), 2-fluorobenzaldehyde (5) and 1-(4-methylphenyl)thiourea (3), as shown in Scheme 1. The compound (6) was obtained with a high degree of purity and yield (66%). The purification processes involved only recrystallization in ethanol. The compound (3) 1-(4-methylphenyl)thiourea was obtained in accordance with the protocols previously published [4].
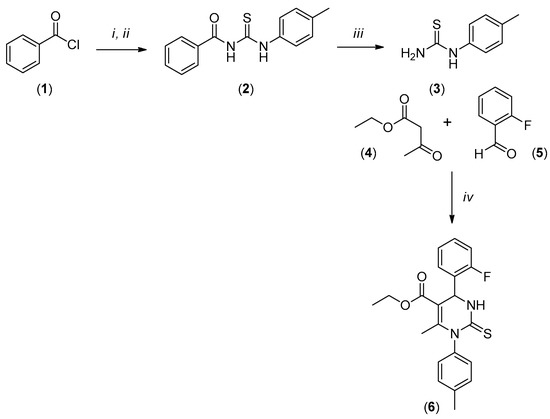
Scheme 1.
Reactions and conditions: i NH4SCN (Me)2CO 60 °C, 15 min; ii 4-MePhNH2, (Me)2CO, 60 °C, 30 min; iii NaOH aq 2.5 M 90 °C, 20 min; iv TMSCl, DMF, r.t, 72 h.
The structural elucidation of the compound (6) was made based on spectroscopic data, and the results are displayed in the experimental section and in the electronic supporting information. From the 1H-NMR spectra (Figure S1), one broad singlet of the NH-3 proton of the DHPM core appeared at 7.53 ppm. Aromatic hydrogens of the two rings produced the next group of signals at 6.91–7.34 ppm. The benzylic hydrogen produced a signal at 5.72 ppm, while the singlet of allylic CH3 resonated at 2.18 ppm. The signals that appeared as a triplet and quartet at 1.14 and 4.08 ppm, respectively, corresponded to the H3CCH2 system. The methyl group linked to the aromatic ring at N1 produced a signal at 2.40 ppm.
In the APT 13C-NMR spectrum (Figure S2), the most representative signals were the methyl carbons at 14.0 and 18.6 ppm, the methyne carbon at 49.6 ppm, and the methylene carbon at 60.6 ppm. The ester carbonylic carbon produced a signal at 165.5 ppm, while the most downfield signals, which appeared at 178.8 ppm, corresponded to the quaternary carbon of the C=S bond carbonyl, which are situated between the two nitrogens. In addition, the ipso, ortho, meta, and para couplings between 19F-13C were identified. The expansion of the aromatic region allowed for the calculation of the coupling 19F-13C constants and the assignment of the aromatic carbons of the ring linked to position 4 of the DHPM core.
3. Materials and Methods
3.1. Chemical Analysis
All the chemicals were purchased as reagent grade and used without further purification. Melting points were determined on a Fisatom 431 apparatus, and were uncorrected. Nuclear magnetic resonance spectra of carbon and proton were recorded in a Bruker Ascend NMR with standard pulse sequences operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR using CDCl3 as a solvent. FT-IR spectra was obtained in a Perkin Elmer spectrometer, opening in ATR mode.
3.2. Synthesis of 1-(4-Methylphenyl)thiourea
For a solution of ammonium thiocyanate (1.1. equiv) in dry acetone at room temperature, benzoyl chloride (1 equiv) was added and the solution was heated at 70 °C for 15 min. Following this, p-toluidine (1 equiv) in acetone was dropwise added and heating of the solution was maintained for 30 min. The reactional medium was poured into water at room temperature and the precipitate was filtered and submitted to alkaline hydrolysis in NaOH 2.50 M, 90 °C for 20 min. Following this, the pH of the system was adjusted to 2 with HCl 37%, and 8 with NH4OH 28%. The product was filtered and presented a high level of purity
3.3. Synthesis of Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate–LaSOM 282
A mixture of ethyl acetoacetate (1 equiv), 1-(4-methylphenyl)thiourea (1 equiv) and a substituted benzaldehyde (1 equiv) were solubilized in DMF under ultrasound for 1 h. TMSCl (6 equiv) was then added dropwise and the mixture was stirred at room temperature for 72 h. After the mixture was poured over three volumes of water, it was submitted to ultrasound for one hour and the precipitate obtained was filtered and washed with water. The crude isolate was recrystallized from ethanol.
Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (6): melting point: 226–230 °C; yield: 66%, recrystallized from ethanol. RMN 1H (CDCl3, 400 MHz, δ ppm): 1.14 (t, 3J = 7.1 Hz, 3H); 2.18 (s, 3H); 2.40 (s, 3H); 4.08 (q, 3J = 7.1 Hz, 2H); 5.72 (d, 3J = 3.1 Hz, 1H); 7.00 (s, 1H); 7.06–7.20 (m, 3H); 7.22–7.34 (m, 4H); 7.53 (d, 3J = 2,8 Hz, 1H). RMN 13C (CDCl3, 100 MHz, δ ppm): 14.0; 18.6; 21.3; 49.6; 60.6; 104.0; 116.2 (d, 2J = 21.7 Hz); 124.5 (d, 4J = 3.4 Hz); 128.4; 128.4; 128.5 (d, 2J = 13.7 Hz); 130.2; 130.3; 138.1; 138.8; 147.7; 160.9 (d, 1J = 247.6 Hz), 165.3; 178.8. HRMS (m/z): calcd. C21H21FN2O2S [M + H]+: 385.1381, found 385.1437. FT-IR (cm−1): 3192 (NH); 1704 (C=O); 1629 (C=C); 1164 (C-O).
Supplementary Materials
The following are available online: FT-IR, 1H-NMR, 13C-NMR, and HRMS spectra for product 6.
Author Contributions
Synthesis NMR spectra obtaining, I.L.G, L.D., and A.F.S; Design of compound, L.P.K, G.M.N., and V.L.E.; Writing—original draft preparation, I.LG, L.P.G, G.M.N., S.C.G., and V.L.E.; Writing—review, S.C.G., and V.L.E.; Supervision and project administration, V.L.E.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Brazilian funding agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for their financial support. The authors wish to thank Professor Rômulo Faria Santos do Canto for obtaining the HRMS spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Davi, L.; Rockenbach, L.; das Neves, G.M.; Kagami, L.P.; Canto, R.F.S.; Figueiró, F.; Battastini, A.M.O.; Eifler-Lima, V.L. Versatility of the Biginelli reaction: Synthesis of new biphenyl dihydropyrimidin-2-thiones using different ketones as building blocks. Tetrahedron Lett. 2018, 59, 2759–2762. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Rockenbach, L.; das Neves, G.M.; Göethel, G.; Nascimento, F.; Kagami, L.P.; Figueiró, F.; de Azambuja, G.O.; de Fraga Dias, A.; Amaro, A. Effect of N-1 arylation of monastrol on kinesin Eg5 inhibition in glioma cell lines. MedChemComm 2018, 9, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Corbelini, P.F.; Figueiro, F.; das Neves, G.M.; Andrade, S.; Kawano, D.F.; Oliveira Battastini, A.M.; Eifler-Lima, V.L. Insights into Ecto-5′-Nucleotidase as a New Target for Cancer Therapy: A Medicinal Chemistry Study. Curr. Med. Chem. 2015, 22, 1776–1792. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, F.; Mendes, F.B.; Corbelini, P.F.; Janarelli, F.; JANDREY, E.H.F.; Russowsky, D.; Eifler-Lima, V.L.; BATTASTINI, A.M.O. A Monastrol-derived Compound, LaSOM 63, Inhibits Ecto-5’Nucleotidase/CD73 Activity and Induces Apoptotic Cell Death of Glioma Cell Lines. Anticancer Res. 2014, 34, 1837–1842. [Google Scholar] [PubMed]
- Schüller, A.; Hähnke, V.; Schneider, G. SmiLib v2. 0: A Java-Based Tool for Rapid Combinatorial Library Enumeration. QSAR Comb. Sci. 2007, 26, 407–410. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, H.; Li, H. SHAFTS: A hybrid approach for 3D molecular similarity calculation. 1. Method and assessment of virtual screening. J. Chem. Inf. Model 2011, 51, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).