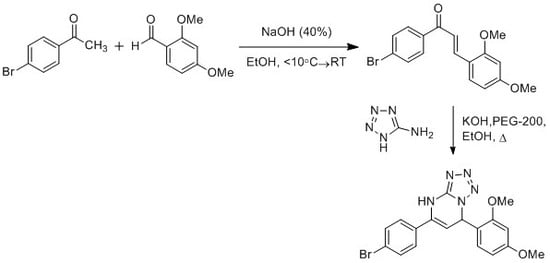
5-(4-Bromophenyl)-7-(2,4-dimethoxyphenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General
3.2. Synthesis of Chalcone Derivative 1
3.3. Synthesis of Target Compound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. Arkivoc 2012, 66–133. [Google Scholar] [CrossRef]
- de Fátima, Â.; Braga, T.C.; da S. Neto, L.; Terra, B.S.; Oliveira, B.G.F.; da Silva, D.L.; Modolo, L.V. A mini-review on Biginelli adducts with notable pharmacological properties. J. Adv. Res. 2015, 6, 363–373. [Google Scholar] [CrossRef]
- Mohana Roopan, S.; Sompalle, R. Synthetic chemistry of pyrimidines and fused pyrimidines: A review. Synth. Commun. 2016, 46, 645–672. [Google Scholar] [CrossRef]
- Hussein, A.M. Synthesis of some new purine-related compounds: Regioselective one-pot synthesis of new. J. Saudi Chem. Soc. 2010, 14, 61–68. [Google Scholar] [CrossRef]
- Raju, C.; Madhaiyan, K.; Uma, R.; Sridhar, R.; Ramakrishna, S. Antimicrobial and antioxidant activity evaluation of tetrazolo[1,5-a] pyrimidines: A simple diisopropylammonium trifluoroacetate mediated synthesis. RSC Adv. 2012, 2, 11657–11663. [Google Scholar] [CrossRef]
- Bahashwan, S.A.; Fayed, A.A.; Amr, A.E.G.E.; Flefel, E.M.; Kalmouch, A. Synthesis and pharmacological activities of some new triazoloand tetrazolopyrimidine derivatives. Molecules 2013, 18, 15051–15063. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A.M.; Guo, H.; Westby, G.; Liu, Y.; Simsek, E.; Guo, J.T.; Mehta, A.; Norton, P.; Gu, B.; Block, T.; et al. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob. Agents Chemother. 2007, 51, 4427–4437. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Synthesis and biological evaluation of novel 1,2-naphthoquinones possessing tetrazolo[1,5-a]pyrimidine scaffolds as potent antitumor agents. RSC Adv. 2015, 5, 24960–24965. [Google Scholar] [CrossRef]
- Hashim, J.; Arshad, N.; Khan, I.; Nisar, S.; Ali, B.; Iqbal Choudhary, M. Preparation of dihydrotetrazolo[1,5-a]pyrimidine derivatives from Biginelli 3,4-dihydropyrimidine-2-thiones. Tetrahedron 2014, 70, 8582–8587. [Google Scholar] [CrossRef]
- Orlov, V.D.; Desenko, S.M.; Pivnenko, N.S. Synthesis and tautomerism of 5,7-diaryl-4,7(6,7)-dihydrotetrazolo[1,5-a]pyrimidines. Khim. Geterotsikl. Soedin. 1989, 3, 1518–1533. [Google Scholar] [CrossRef]
- Kour, P.; Singh, V.P.; Khajuria, B.; Singh, T.; Kumar, A. Al(III) chloride catalyzed multi-component domino strategy: Synthesis of library of dihydrotetrazolo[1,5-a]pyrimidines and tetrahydrotetrazolo[1,5-a]quinazolinones. Tetrahedron Lett. 2017, 58, 4179–4185. [Google Scholar] [CrossRef]
- Suwito, H.; Kurnyawaty, N.; Ul Haq, K.; Abdulloh, A.; Indriani, I. Ethyl 5-methyl-7-(4-morpholinophenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate. Molbank 2018, 2018, M998. [Google Scholar] [CrossRef]
- Suwito, H.; Pudjiastuti, P.; Fanani, M.Z.; Kimata-ariga, Y.; Katahira, R.; Kawakami, T.; Fujiwara, T.; Hase, T.; Sirat, H.M.; Nyoman, N.; et al. Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin—Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents. Molecules 2014, 21473–21488. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef] [PubMed]


| No. Atom | δH (ppm), mult, J (Hz) | δC (ppm) | HMBC |
|---|---|---|---|
| 1 | 151.1 | ||
| 2 | 10.40 (s, 1H) | C-1, C-4 | |
| 3 | 134.1 | ||
| 4 | 5.17 (d, J = 3.7 Hz, 1H) | 97.3 | C-3, C-5 |
| 5 | 6.64 (d, J = 3.7 Hz, 1H) | 54.6 | C-3, C-4, C-6, C-7, C-11 |
| 6 | 120.6 | ||
| 7 | 157.8 | ||
| 7-OMe | 3.73 (s, 3H) | 55.8 | C-7 |
| 8 | 6.60 (d, J = 2.3 Hz, 1H) | 99.0 | C-9, C-10 |
| 9 | 160.9 | ||
| 9-OMe | 3.75 (s, 3H) | 55.3 | C-9 |
| 10 | 6.52 (dd, J = 8.5, 2.3 Hz, 1H) | 105.2 | C-6, C-8 |
| 11 | 7.04 (d, J = 8.5 Hz, 1H) | 129.0 | C-5, C-7, C-9 |
| 1′ | 122.2 | ||
| 2′, 6′ | 7.56 (d, J = 8.6 Hz, 2H) | 128.0 | C-1′, C-2′/C-6′, C-3 |
| 3′, 5′ | 7.62 (d, J = 8.6 Hz, 2H) | 131.5 | C-1′, C-3′/C-5′, C-4′ |
| 4′ | 133.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, K.U.; Liawati, M.D.; Abdulloh, A.; Suwito, H. 5-(4-Bromophenyl)-7-(2,4-dimethoxyphenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine. Molbank 2018, 2018, M1037. https://doi.org/10.3390/M1037
Haq KU, Liawati MD, Abdulloh A, Suwito H. 5-(4-Bromophenyl)-7-(2,4-dimethoxyphenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine. Molbank. 2018; 2018(4):M1037. https://doi.org/10.3390/M1037
Chicago/Turabian StyleHaq, Kautsar Ul, Mareta Dewi Liawati, Abdulloh Abdulloh, and Hery Suwito. 2018. "5-(4-Bromophenyl)-7-(2,4-dimethoxyphenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine" Molbank 2018, no. 4: M1037. https://doi.org/10.3390/M1037