1. Introduction
Individual identification of free-living animals allows one to differentiate among individuals of a species and estimate the size of its population. One of the most commonly used procedures is the capture-recapture of individuals based on the Lincoln-Petersen method [
1]. In a first sample, animals are captured, marked and released, and subsequently another sample of the population is captured. The proportion of individuals marked in the second sample permits the estimation of the total population size [
2]. This basic model form was expanded to allow testing of various hypotheses about the factors influencing capture probability [
3] and more recently to allow the incorporation of spatial information [
4] yet the need for “capture” remains in all cases. Physical capture (and recapture) of individuals is not only invasive and potentially dangerous, but can be challenging or impossible in the case of many large and/or elusive mammals [
5]. Fortunately non-invasive approaches exist for “capture” and “marking” of animals, and photo-identification, often through the use of motion/heat activated trail cameras, has been widely used in a capture-mark-recapture context to estimate population sizes (e.g., Testé and Denis [
6]; Burton et al. [
7]).
From photographic records, it is sometimes possible to characterize specific phenotypic patterns in an individual animal. In the case of mammals, some species have spots, rosettes or stripes on the coat, which are unique features and do not change with time [
8,
9]. As a result, photographic records can represent marking events, with subsequent photos representing recaptures. These photo records can then be analyzed to determine the number of different individuals of a species that were not only photographed but also missed. The information obtained from the individual identification of animals is essential in the study of animal behavior and population demographics [
10].
There are examples in the literature of individual identification of wild mustelids (Carnivora: Mustelidae). Trujillo et al. [
11] described individuals of the giant otter (
Pteronura brasiliensis) with a uniquely patterned white-yellow chest patch. Magoun et al. [
12] identified individuals of wolverine (
Gulo gulo) through the observation of the unique light-colored patterns on the chest, throat, and chin area. Sirén et al. [
13] recognized and contrasted individuals of American marten (
Martes americana) through the analysis of the ventral patch on the chest and throat. Harrison [
10] analyzed the pattern of the dorsal head stripes of the American badger (
Taxidea taxus), identified individuals in the wild, and subsequently verified the difference of these characters among preserved individuals in a zoological collection.
The tayra (
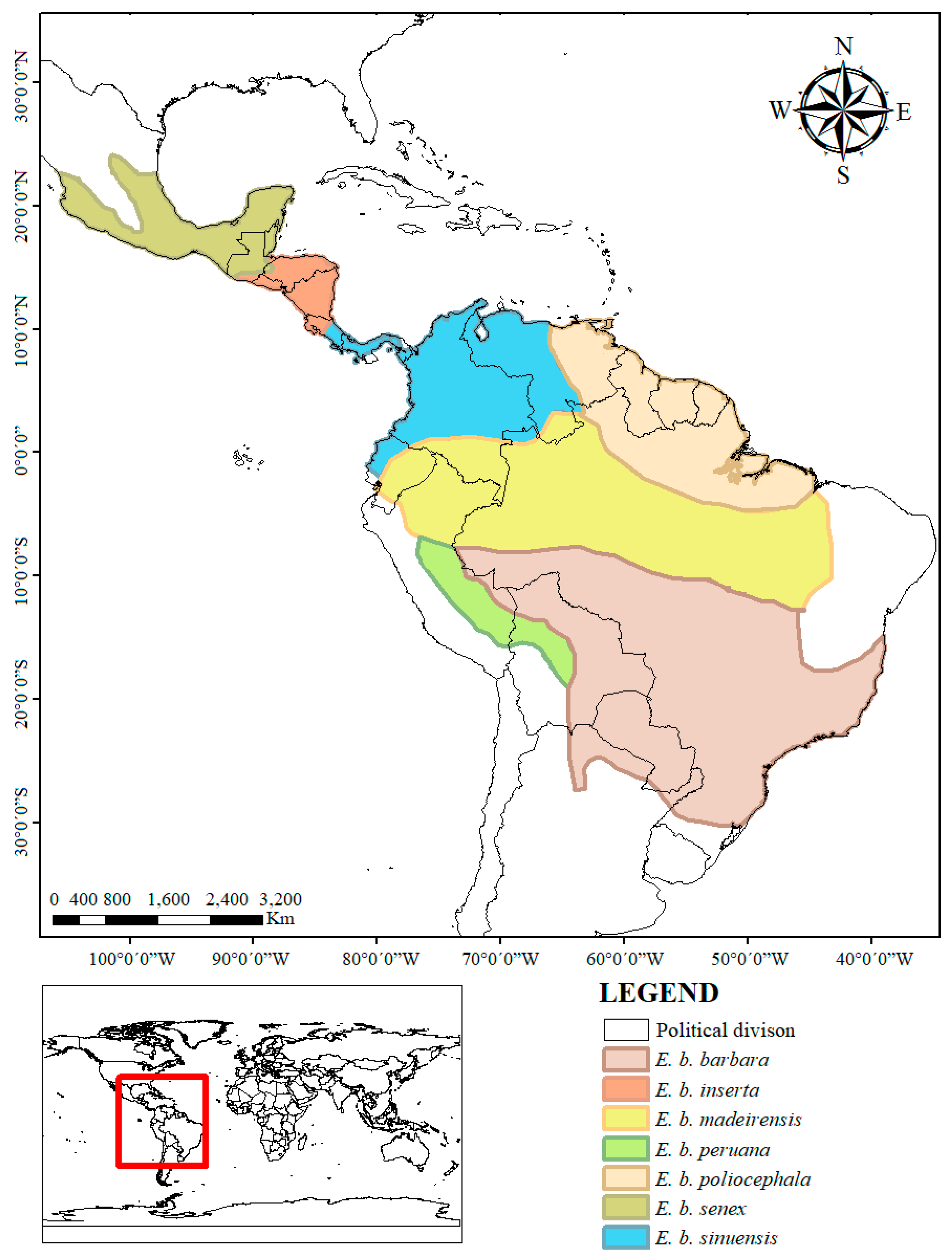
Eira barbara) is a neotropical scansorial (with terrestrial and arboreal habits) mustelid with a distribution that extends from the coasts of Central Mexico to northern Argentina (
Figure A1). This species has variable coat color across its distribution that ranges from uniform (
Figure 1a) to disruptive (
Figure 1b). Seven subspecies based on coat coloration variation have been recognized throughout its range; three for Mexico to Panama, and the rest for South America (
Table 1; [
14,
15,
16]). Recent research based on analysis of mitochondrial DNA has reduced the number of subspecies in South America from five to two [
17]. The exact number of subspecies remains questionable with their current geographical distribution and the status of their populations unknown.
Eira barbara has been little studied and current records of the species include sightings, footprints and/or photographs through camera trapping, but are collateral results of research focused on other species [
18,
19,
20,
21,
22,
23,
24].
Methods to identify individuals of
E. barbara have yet to be reported. To date the identification of a specimen through photographic capture has only related to sex, provided the genitals of the organism are observed (e.g., Ramírez-Bravo [
20]). The identification at the individual level using the phenotypic characteristics of
Eira barbara is complicated. The color of the pelage of the head, neck, trunk, limbs, and tail is very similar, although in some geographic areas the tonality of the hair of the head and neck is different to the rest of the body. However, most populations of
E. barbara have a distinct throat patch (
Table 1), similar to that reported for other mustelids, where the feature has been successfully used for individual identification [
11,
12,
13]. To date it has not been demonstrated whether throat patch variation in
Eira barbara may allow discrimination between individuals, and whether the utility of the throat patch for this purpose varies geographically across the species’ range.
The objective of the present study was to characterize the morphological variability of the throat patch of different museum specimens of Eira barbara collected throughout its range to determine if the throat patch character allows for the identification of individuals of this species. In addition, we aimed to investigate the applicability of our findings in a field setting, by attempting to identify individuals from camera trap photos collected in the Peruvian Amazon.
2. Materials and Methods
We made a review of the zoological collections of the American continent that could contain specimens of Eira barbara, and we contacted the curators to request permission to access their collections. Subsequently we visited the collections that had the largest number of specimens, and the Mexican collections that granted their authorization to examine the specimens. We recorded the collection information available for each specimen, and grouped each specimen by country of collection.
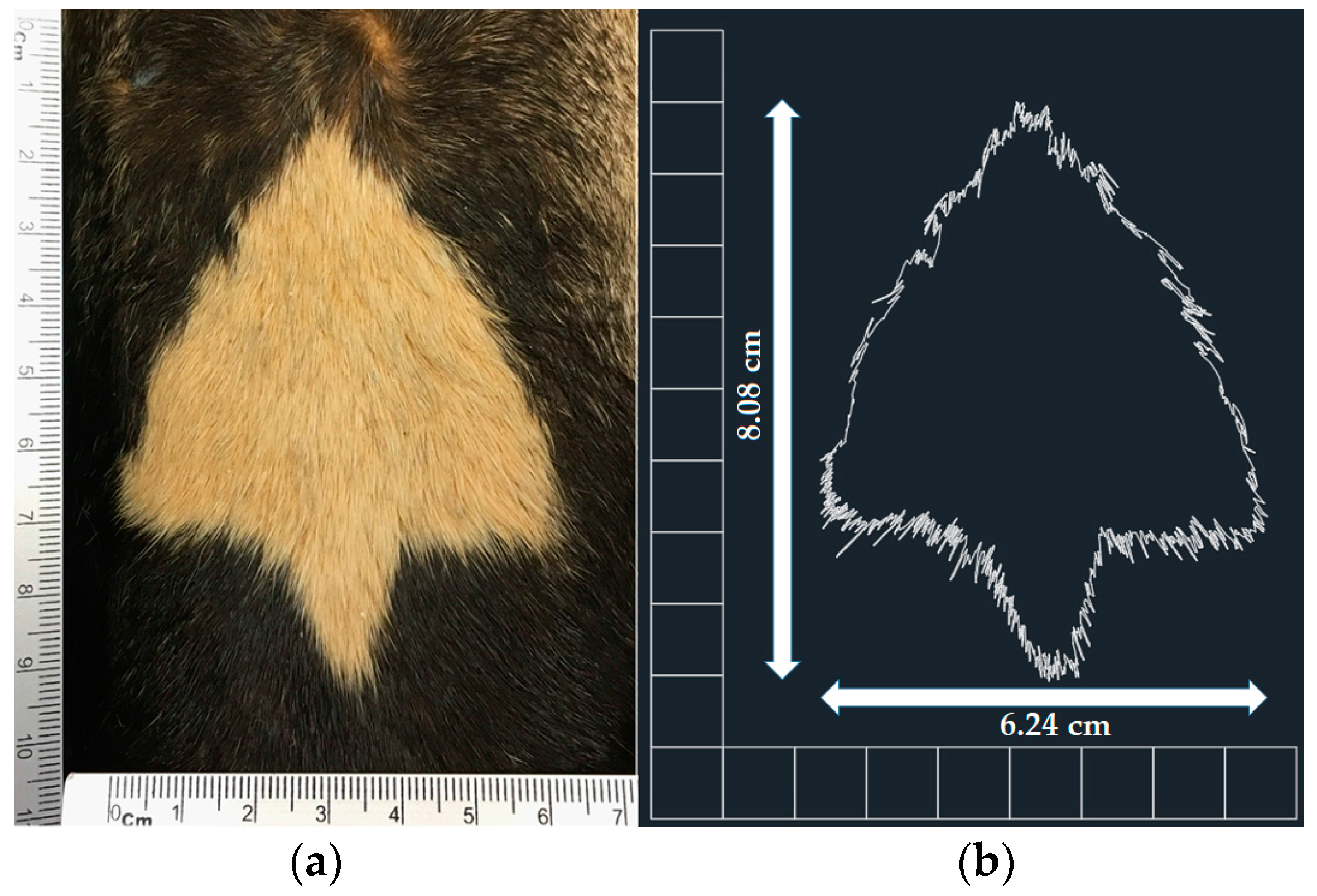
We photographed the throat patch of each of the specimens at a consistent distance (10 cm;
Figure 2a), with a ruler visible to allow setting of the correct scale in the photographs taken. We analyzed the images using AutoCAD software (version 2016), in which the outline of each throat patch was delineated. To identify the length of the patch we measured the distance between the top vertical vertex and the lower vertical side, we obtained the width of each throat patch by measuring the maximum distance between the opposing horizontal vertices (
Figure 2b). Finally, we obtained the area and perimeter of each patch.
Because shape is a difficult parameter to quantify concisely in a metric [
25], we used a Shape Index to characterize throat patch shapes, with calculations based on the relationship between the area and perimeter of a polygon, which facilitates the understanding of a factor at the morphological and functional level [
26]. We used the modification of the Patton’s Index [
27] made by McGarigal and Marks [
25] using the formula:
where p
i is the perimeter (m) of the patch i, and a
i is the area (m
2) of the patch i, the formula can be read as: shape equals patch perimeter (m) divided by the square root of patch area (m
2), adjusted by a constant to adjust for a circular standard. Thus, although patches may possess very different shapes, they may have identical areas and perimeters and shape indexes. For this reason, this shape index is best considered as a measure of overall shape complexity that compares the complexity of a patch shape to a standard shape. In the vector version of FRAGSTATS (version 2.0, McGarigal and Marks [
25]), patch shape is evaluated with a circular standard, with the index referenced as a minimum (1) for circular patches and increasing as patches become increasingly noncircular [
25].
A Wild Population Case Study
To determine the usefulness of potential identification criteria in wild populations, we analyzed independent photographic events of
Eira barbara obtained in the Peruvian Amazon between April and September of 2008. These photographs were the product of a survey designed to estimate density of
Leopardus pardalis [
28] in which 23 camera stations were established, each with two Reconyx RC-55 (Holmern, WI, USA) digital infrared trail cameras placed in lowland tropical rainforest. The sampling effort was of 3068 camera-nights, and the stations formed a polygon of 22 km
2. Cameras were set to take three photos per trigger on the “rapidfire” setting, which allows approximately one photo to be taken per second. The photographs obtained from
Eira barbara separated by 24-h cycles were considered as independent events. Photographs were reviewed in which the position of the organism allowed observation of the throat patch and the images were grouped according to the angle of observation: (a) frontal catches, (b) left side capture and (c) right side capture. Individual identification was attempted for all photographs showing any angle of the throat patch, yet additional characteristics including the presence of testes, ear shape, and overall coat coloration were used to confirm identifications when necessary.
3. Results
A total of 275 museum specimens of
Eira barbara were available for examination; 15 specimens belonged to six zoological collections of Mexico, 103 records belonged to the Division of Mammals of the National Museum of Natural History of the Smithsonian Institution, and 157 specimens were from the zoological collection of the American Museum of Natural History of the United States (
Table 2).
The records of the 275 specimens of
Eira barbara were grouped from the country where they were collected; ten records from Zoo specimens (unknown origin) were grouped in the category named Zoo. Among the analyzed specimens, we found eight specimens whose phenotypic characteristics do not match the description of any taxonomic group currently recognized; these specimens have a white or yellow pelage over all the body with a black snout. They were not included in our detailed analysis because two specimens had a stitched throat patch, and of the remaining six specimens, three had no throat patch and three others were incomplete. The specimens studied were classified into different categories (
Figure 3).
Of the 275 specimens examined, 222 specimens of
Eira barbara had a throat patch yet only 81 patches were complete. Eight of these 81 samples were excluded because the throat patch extended through the neck, shoulder and back; in these cases the throat patches did not have a limit (
Figure 3b) and were impossible to measure. Therefore, only 73 throat patches were used to characterize morphological measurements and the shape index (
Table 3 and
Table A1).
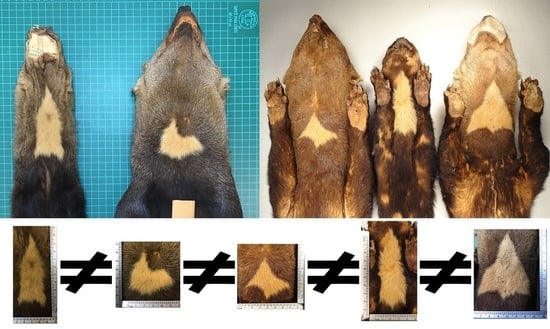
The contours of the throat patches examined were an irregular polygon form, which differed in the number of sides and vertices that composed them. As a result, all the throat patches lacked an axis of symmetry.
The morphological measurements of the 73 throat patches differed in the length, width, area, and perimeter that occupy each patch. The length values ranged from 0.96 to 14.70 cm (throat patches S50 and S27 respectively,
Table A1) with a mean length of (6.03 ± 3.03 cm). The average width of the 73 analyzed patches was (4.31 ± 1.98 cm) with the values ranging from 0.58 to 8.38 cm (patches A1 and S17 respectively,
Table A1). There was a significant positive correlation (
p = 0.76) between the length and width values of the 73 patches (
Figure 4).
The area of the throat patches varied between 0.15 cm
2 and 49.90 cm
2 (A1 and S27 respectively,
Table A1) with an average area of (13.76 ± 11.18 cm
2). The perimeter values ranged from 9.28 to 441.98 cm (patches A1 and A2,
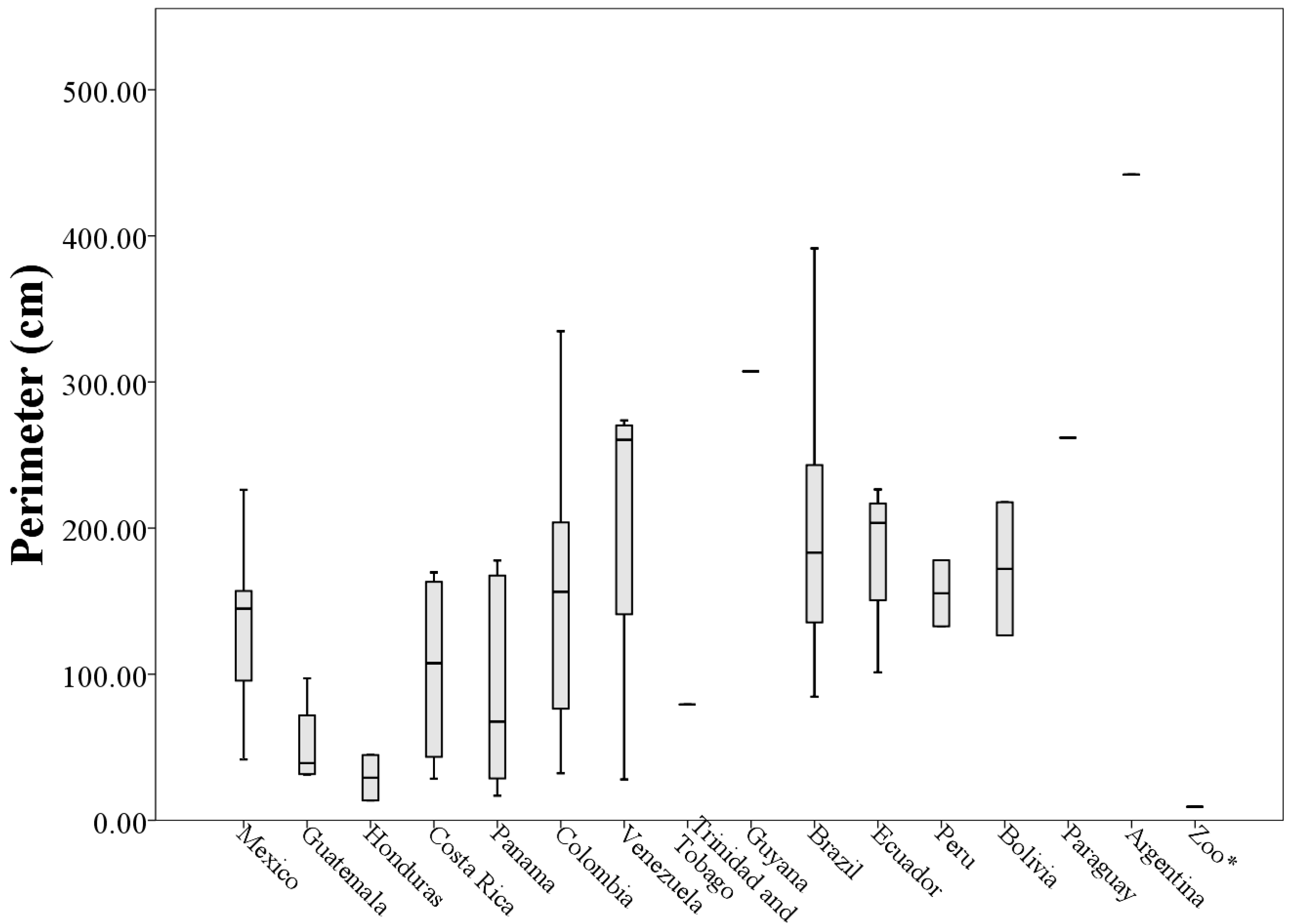
Table A1) and the average was (143.14 ± 92.41 cm).
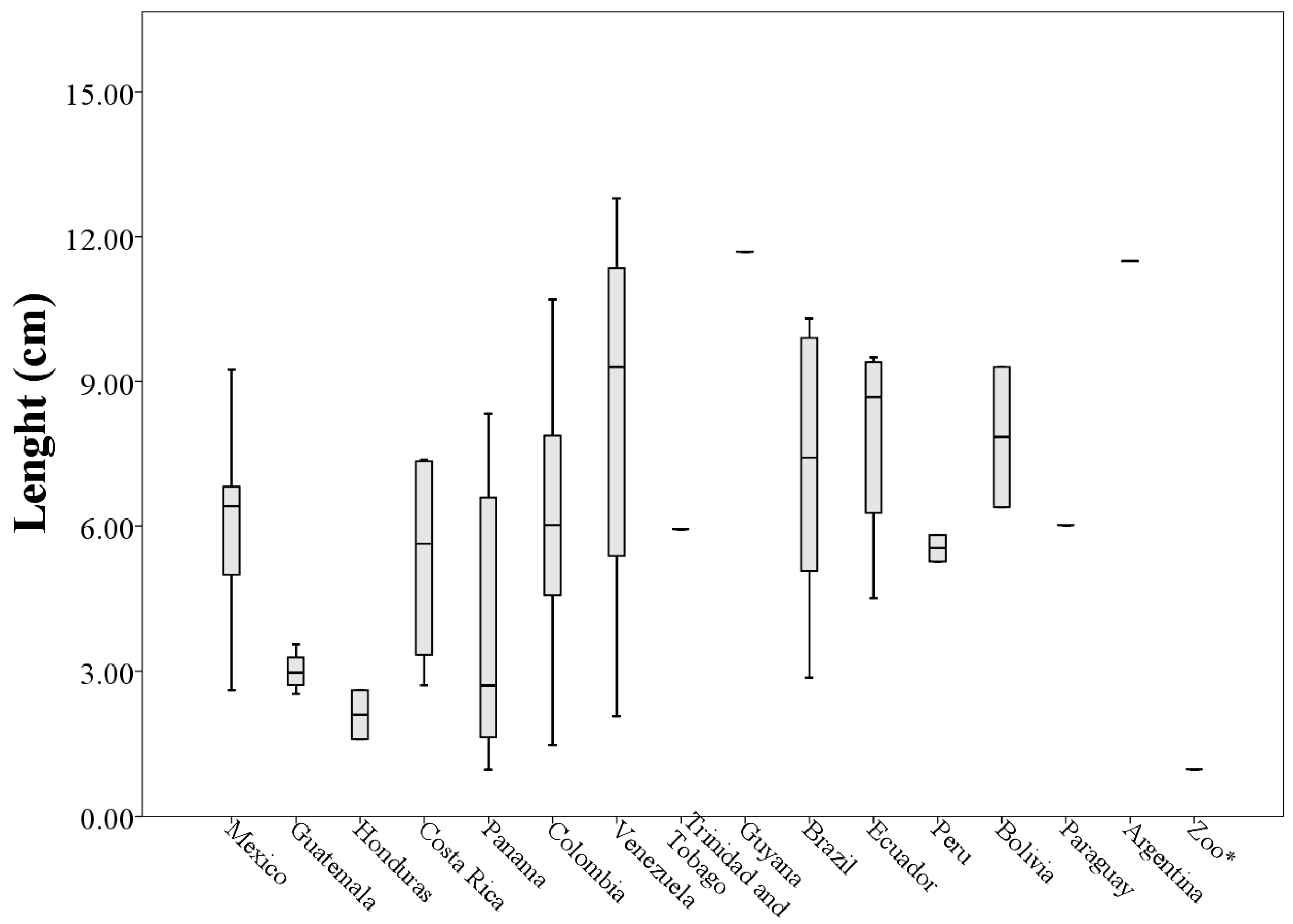
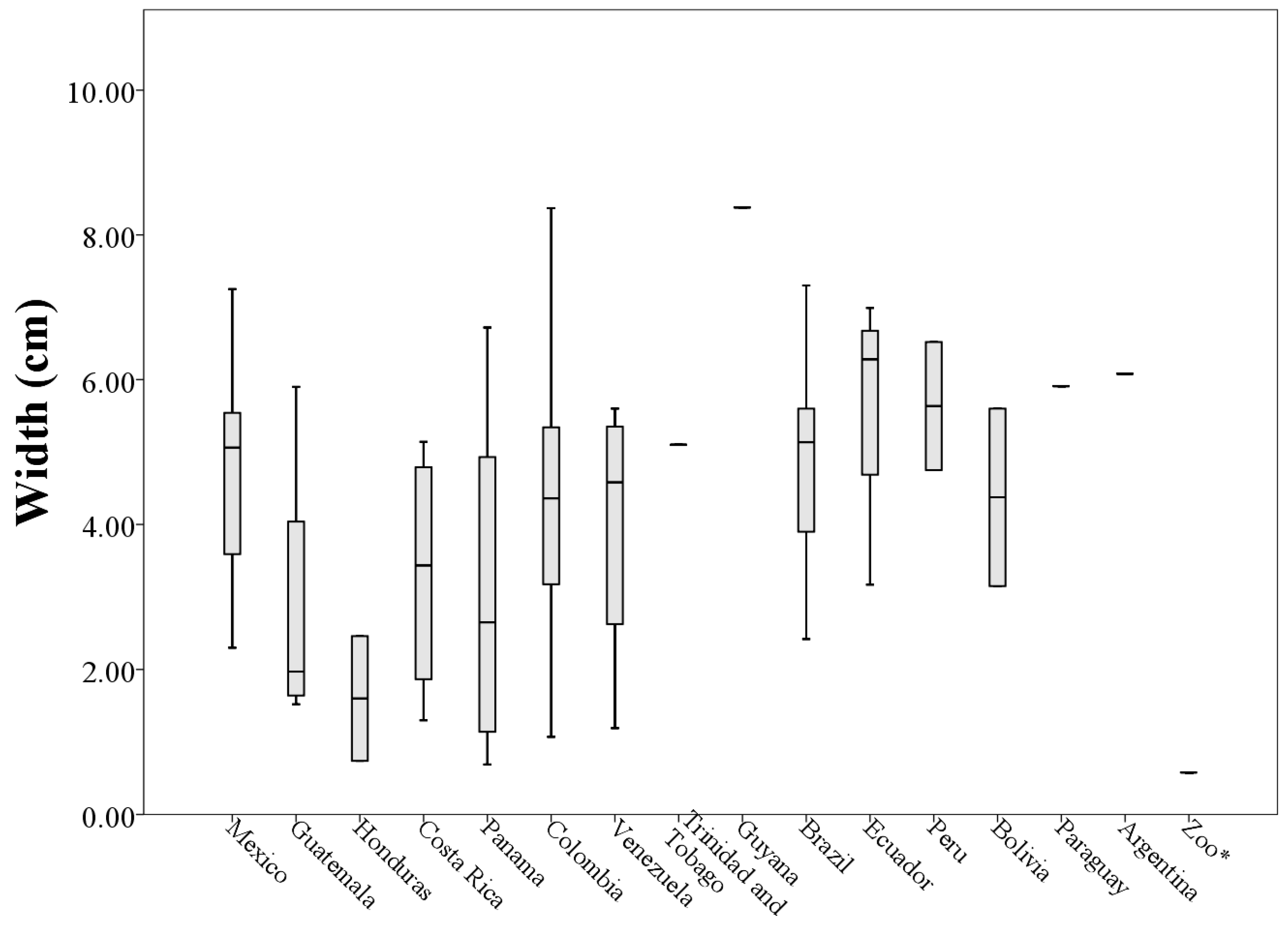
Analyzing morphological measurements of throat patches of specimens grouped by country of origin, the averages of the measures indicated that the longest throat patches belonged to specimens collected in Venezuela (8.36 ± 4.53 cm) and the smallest to specimens collected in Honduras (2.10 ± 0.72 cm). This situation was repeated with the measures of perimeter (205.63 ± 118.73 cm and 29.15 ± 21.93 cm respectively). The patches with the largest values of width and area corresponded to specimens collected in Ecuador (5.68 ± 1.70 cm and 21.80 ± 11.54 cm
2 respectively) and the smallest corresponded to specimens collected in Honduras, (1.60 ± 1.21 cm and 1.22 ± 0.98 cm
2 respectively,
Table 4).
When plotting the values of the morphological measurements of the throat patches grouped by country of origin, geographic variation in the values of length, width, area and perimeter are evident (
Figure 5,
Figure 6,
Figure 7 and
Figure 8). These data, taken along with clear differences in patch shape demonstrate large amounts of variation in patch characteristics both within and among countries (
Figure A2).
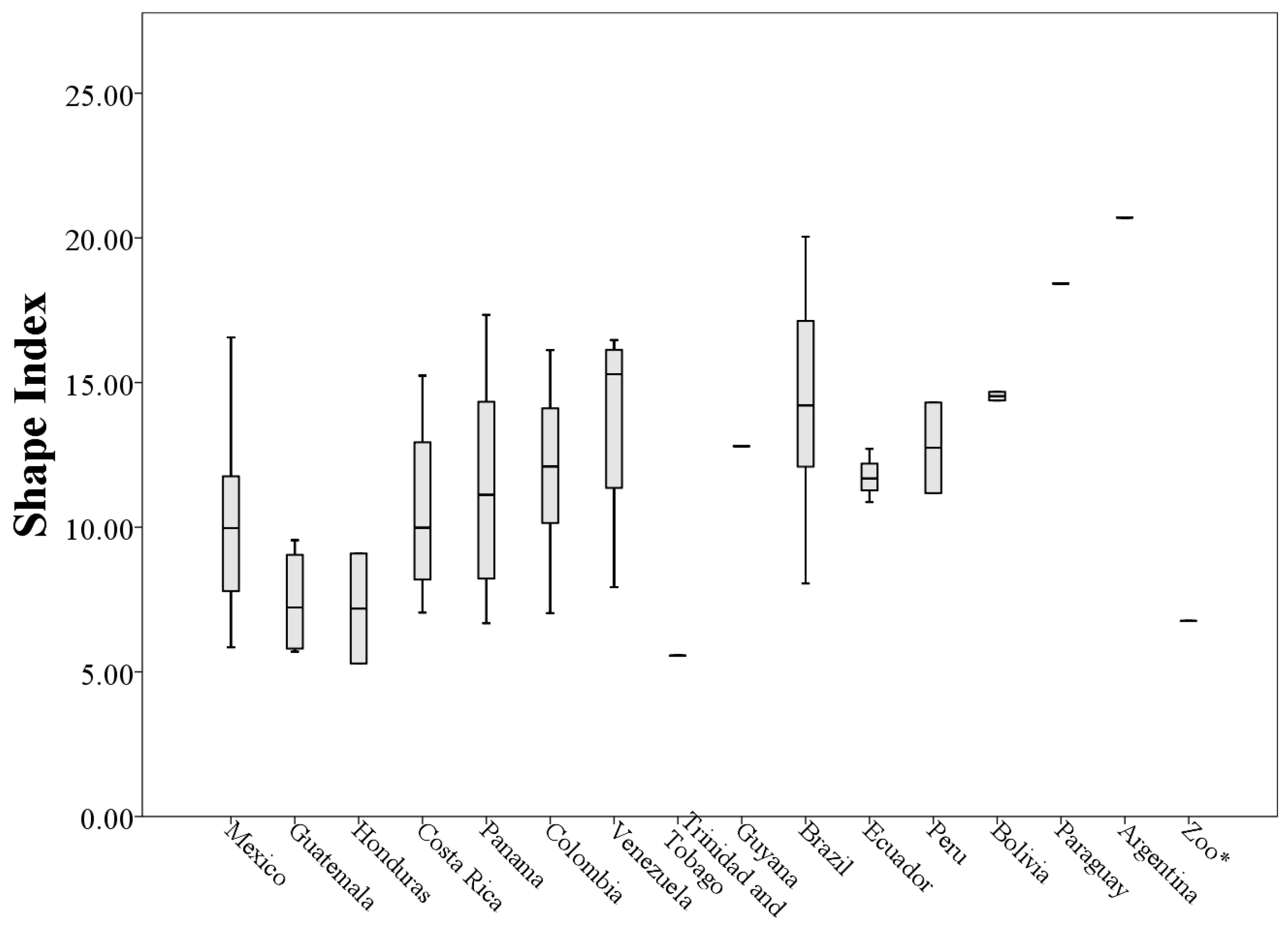
The 73 values of the shape index ranged from 5.29 to 20.70 (minimum and maximum respectively,
Table A1), indicating that the throat patches have an irregular non-circular shape (
Figure 9).
A Wild Population Case Study
We registered 35 independent photographic events with
Eira barbara in the Peruvian Amazon. In 19 events (54.0%) a clear image was obtained of the throat patch, which was subsequently used to identify individuals. For all 19 of these events, we were able to assign an individual to the event. In total, nine different individuals were identified: four males and five animals of indeterminate sex (
Figure 10,
Figure A3). Two individuals were photographed on different dates; individual B eight times and individual F four different times. Both of these individuals were recaptured only in the same camera-station. The other seven individuals were photographed only on a single occasion (
Table 5). In two independent photographic events more than one individual was pictured; in one event two tayras were pictured and another included three in the same photograph.
In the photographs where the view is frontal (
Figure 10, individuals a–d) it is observed that the contour and size (the space occupied by the patch in the gular area of the animal) of the four throat patches were different from each other. For the remaining individuals, only lateral views of the left (
Figure 10, individuals e–g) or the right side (
Figure 10, individuals h,i) were available and individual identification was still possible. However in some cases, particularly with individuals showing only lateral patch views, and where photo angles across different photo events were substantially different (e.g.,
Figure 10e–g), additional characteristics, such as coat color variation and ear shape, were critical in confirming final identifications. Differential coat coloration between the neck and body was seen in 77.7% of the individuals (e.g.,
Figure 10e).
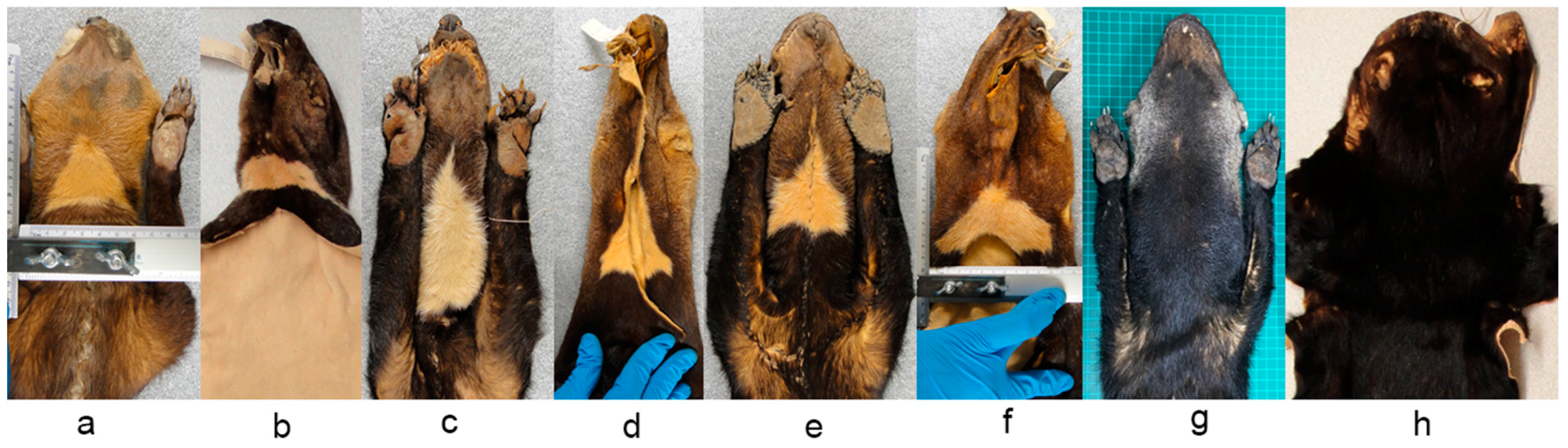
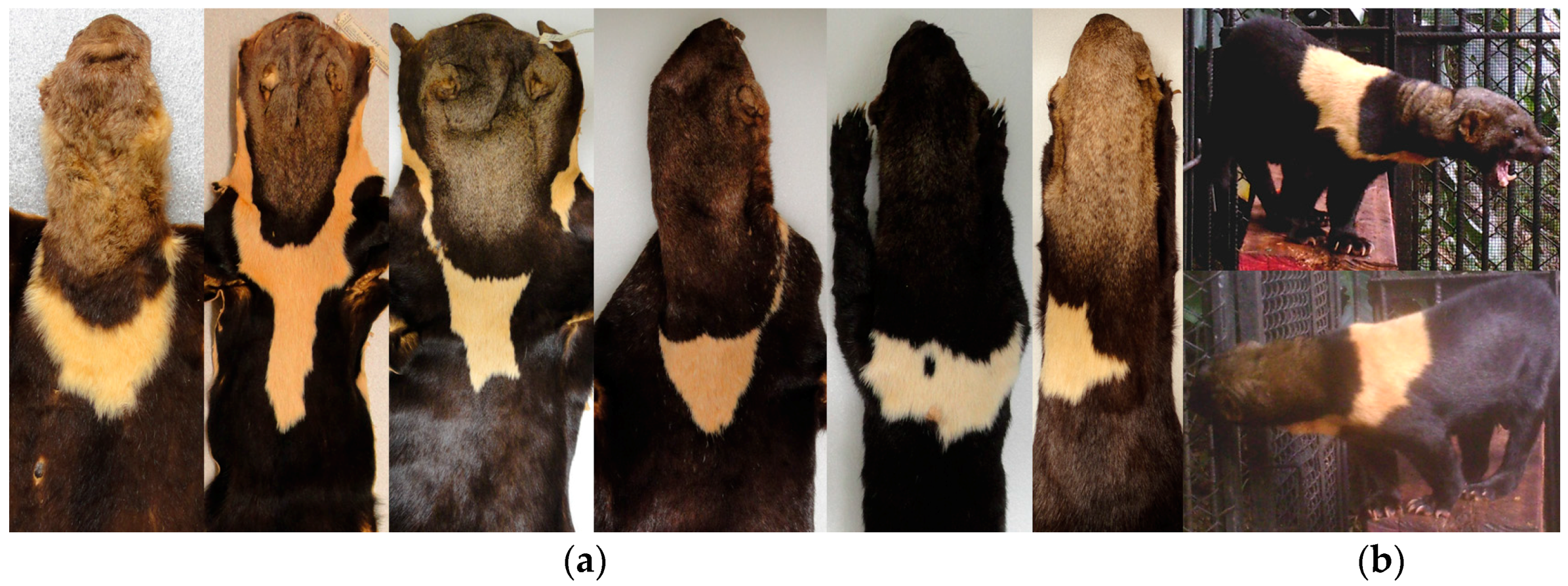
Some museum specimens were not included in our detailed analysis because the throat patch were incomplete or artificially matched (i.e., stitched), and the coat color had particular characteristics which made them stand out from the others. In the case of the subspecies
Eira barbara poliocephala the throat patch extends to the shoulders and back. In the specimens we examined it was evident that the form of this character also differs between organisms in this group (
Figure 11a). It was observed that this character has an asymmetrical outline, and therefore has a different form in left and right lateral planes (
Figure 11b).
Presley [
14] pointed out that a yellow morph of
E. barbara (
Figure 12a) is relatively common in Guyana, and the eight specimens we found with this characteristics were all collected in Guyana. The throat patch was present in two of the eight examined specimens (
Figure 12b), in the remaining six specimens, three had no throat patch (
Figure 12c) and three others were incomplete.
4. Discussion
Quantitative measurements of 73 throat patches have demonstrated that there is sufficient variation in the shape and size of each gular spot is unique in Eira barbara, this character can be used to identify tayras on an individual level. Analysis of camera trap photos from a Peruvian Amazon population has demonstrated that it is possible to individually identify tayras with this non-invasive method. The individual identification of tayras using the throat patch can be applied throughout the tayra range and for all the coat color variation in the species, including the white/yellow morphs and those with disruptive coat color.
The descriptions of the subspecies recognized by Cabrera [
15] and Hall [
16] reflect the phenotypic variability of
Eira barbara through their geographical distribution. These descriptions are based on an arbitrary and subjective analysis of qualitative characters as opposed to genetic analysis. According to Avise and Ball [
29], the subspecies designation should be made based on concordant distributions of multiple independent (genetic) traits. Research conducted by Ruiz-García et al. [
17] was focused on generating a phylogenetic reconstruction between
Potos flavus and
Eira barbara. They analyzed biological samples of 68 specimens collected in South America and grouped them according to the five recognized subspecies in that region (
barbara,
sinuensis,
peruana,
madeirensis and
poliocephala) according to Cabrera [
15] and Hall [
16]. Molecular results suggest that in South America there are only two subspecies of
Eira barbara:
barbara (formed by groups
barbara,
peruana,
sinuensis and
madeirensis) and
poliocephala. Consequently, the subspecies of
Eira barbara currently recognized are four:
senex,
inserta (the result of phenotypic descriptions),
barbara and
poliocephala (the result of phylogenetic analysis).
Due to the lack of information concerning the coat color variation (regardless of patch characteristics) of
Eira barbara throughout its area of distribution, we recommend a more comprehensive review of the available zoological collections of the world to generate detailed descriptions of the different existing phenotypes and maps of the distribution of each one. It is also necessary to perform the analysis of mitochondrial DNA of
mtCyt-b and
mtNADH-5 of specimens collected in Central America with the aim of completing the investigation of Ruiz-García et al. [
17], and to identify if the populations of
Eira barbara present from Mexico to Panama correspond to the subspecies distributed in South America (
Eira barbara barbara) or if they comprise unique subspecies.
The specimens examined in this research were collected through almost all the area of distribution of the species, and 80.7% (222 of 275) of the examined specimens had a throat patch. However, 30 specimens (10.9%) examined did not have this character, and these were not melanic or albino organisms. Five (1.8%) were collected in Mexico, five in Nicaragua (1.8%), four in Costa Rica (1.4%), nine in Panama (3.2%), four in Venezuela (1.4%) and three in Guyana (1.0%). These results demonstrates that the absence of the throat patch is not restricted to a single population; this condition occurs throughout the northern half of the species range. Regardless, the presence of a clear throat patch in more than 80.0% of examined specimens indicates that this feature will typically be available for individual identification in field populations.
In the case of
Eira barbara, the literature describes atypical coat coloration [
30,
31]. Krumbiegel [
32] argues that the cases of albinism and melanism are most common in
Eira barbara than in any other species of mustelid. The lack of a throat patch may be a recurrent genetic mutation in the coloration of the coat similar to that taking place in melanism, albinism or leucism, which occurs in a small percentage of the population [
33,
34]. These genetic alterations have not been studied in
Eira, but have been investigated in different mammalian species [
24,
35,
36,
37].
Some species of animals have some unique external characteristic which makes the identification of individuals feasible [
38]. The results of this research show that the form (geometric information that results from removing the effects of position, scale and rotation of an object, [
39]) and size of the patch on the throat is a distinctive character in every organism of
Eira barbara. This feature serves as a point of individual reference that allows the identification and differentiation of organisms of
Eira barbara that have a throat patch. Specific patterns in the coat of an animal are unique and do not change over time [
8]. In the case of
Eira barbara, theoretically, the size of the throat patch will increase proportionally until the animal reaches adult size (this occurs at six months of age, [
14]).
Previous research that focused on the individual identification of mustelids (e.g., Magoun et al. [
12], Harrison [
10] and Sirén et al. [
13]) has been based on visual and subjective analysis of the obtained photographic records. Our research was based on the analysis of different quantitative and morphological characteristics of throat patches, which showed that the throat patch is a unique characteristic among individuals, and that it can be used as a basis for the individual identification of wild animals. Ours is the first to combine analysis of camera trap photos with quantitative measurements from a range of museum specimens across the species’ range. This allows us to present conclusions on the potential feasibility of camera trap-based field studies throughout the tayra distribution.
A point in common our work and some previous investigations is that the individual identification is only possible when the photographic record of the animal is in a specific position, which allows detailed observation of the distinctive pattern in the pelage. In the case of
Gulo gulo [
12] and
Martes americana [
13] individual identification is only possible if the front of the gular area is photographed (ventral view), whereas in the case of
Taxidea taxus [
10] it is only necessary to obtain photographs of any side of the head in lateral view. Our results for
Eira barbara show that individual identification is possible with photographs showing the animal’s gular area either with a front (ventral view) or side (lateral view). While a clear ventral view allows unambiguous identification from a single clear photograph, the lateral views require both sides to be photographed for unambiguous identification. In addition, the utility of lateral views is somewhat dependent on the angle of the photograph, and some body positions can obscure the throat patch to varying degrees. This is not the case with individuals of the subspecies
Eira barbara poliocephala, where the throat patch extends over the shoulders and back, and where any lateral view will provide identifiable characteristics for that side. Field studies in Guyana, where the white/yellow morph appears to be common will face additional challenges where our limited sample size indicates the absence of a throat patch may be more common and throat patches, when present, may be more challenging to distinguish from camera trap photographs.
It could be difficult to differentiate individuals if only one side of the tayra is photographed. While it is possible to compare photographs of the same anatomical side of the animal, individual identification could be complicated when comparing different sides, as is the case with jaguars or other individually identifiable species [
40]. Given the non-symmetric forms of the patches, we would recommend that camera trap surveys aimed at estimating tayra populations include two cameras at each station as recommended for other species. In instances where only one camera per station is available, some additional options can improve the chances of unambiguous individual identification. First, the use of additional characteristics such as presence or absence of testicles, coat color variation, and shape of the ears, tail and body can certainly aid identification, and these factors were helpful in the identification of numerous individuals in our Peru field case study. Give this, camera setups that increase the amount of time the individual spends in front of the camera, and the chances that the animal will show clear views throat patch will improve one’s ability to discriminate individuals with certainty. A range of commercial carnivore lures for example, are likely to at least slow the movement of this species and increase the number of angles from which the animal is photographed. In our field study, tayra reacted strongly to a proprietary carnivore lure (Weaver’s Cat Call) and spent significant time investigated a scented stake placed in front of a subset of locations. Previous researchers have also designed custom bait stations which require individuals to expose their gular area to the camera while accessing the bait (e.g., Magoun et al. [
12] and Sirén et al. [
13]).
Newer spatially explicit capture-recapture (SECR) models designed to estimate density require a reasonable number of individuals to be captured on multiple occasions to allow unbiased estimation of capture probability [
41]. While there is no specific guidance on absolute lower thresholds, the nine individuals identified here is likely near the minimum number of individuals required for SECR models. Based on our field study and others, capture rates (independent photo events per 100 camera-nights) for tayra vary widely (this study: 1.14; Campeche, Mexico: 0.67 (Á. J. Villafañe-Trujillo, unpublished data); Iguacu National Park, Brazil: 0.40, [
42]) and are typically similar or below capture rates for jaguar in the same areas (this study, [
43,
44]). Estimation of jaguar densities using camera traps, while common, has been challenging to implement without bias due to low capture rates and large jaguar home ranges [
45]. Given that capture rates of tayra are unlikely to be higher than those seen for jaguars, estimation of tayra density from camera surveys is likely to face similar challenges in accumulating enough individuals over a reasonably closed study period. Because our tayra capture rates appear to be at the higher end of those reported, our camera effort (3068 camera-nights) should be seen as a minimum required to accrue sufficient tayra captures, and in some areas twice this value may be necessary. There is some indication that tayra may be more likely to be captured off trails [
44], and so future studies may consider this option to further increase capture rates. SECR studies also require that a large percentage of captured individuals be captured at multiple camera stations to allow estimation of an animal movement parameter [
41]. As a result, field studies aiming to use SECR to estimate density should aim for a camera spacing that attempts to find a compromise between maximizing the number of individuals captured (i.e., maximizing the size of the study area and capture probability), and maximizing spatial recaptures of individuals (i.e., minimizing camera spacing; [
41,
45,
46]). In this study, cameras were spaced on average 1.1 km apart, and none of the captured tayra were photographed at multiple camera stations, potentially suggesting that tayra in this region have relatively small ranges and that in general, spacing should be substantially less than 1km to ensure spatial recaptures of individuals.