Contrasting Patterns of Pomacea maculata Establishment and Dispersal in an Everglades Wetland Unit and a Central Florida Lake
Abstract
:1. Introduction
2. Materials and Methods
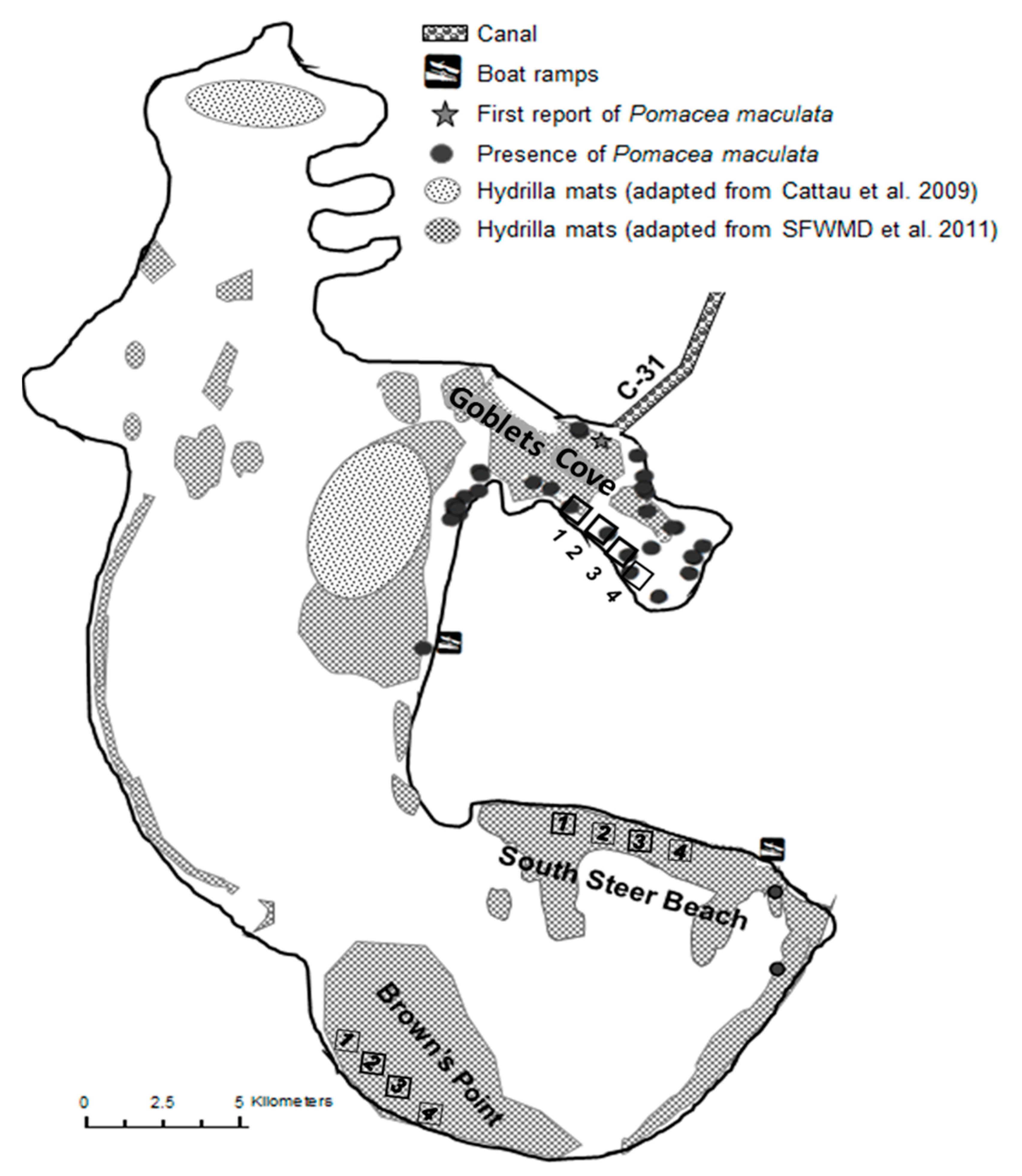
2.1. Study Sites
2.2. Water Depth Data
2.3. Estimates of Apple Snail Density
2.4. Estimates of Egg Presence
2.5. Data Analyses
3. Results
3.1. P. paludosa Occurrence—LTOHO and WCA3A
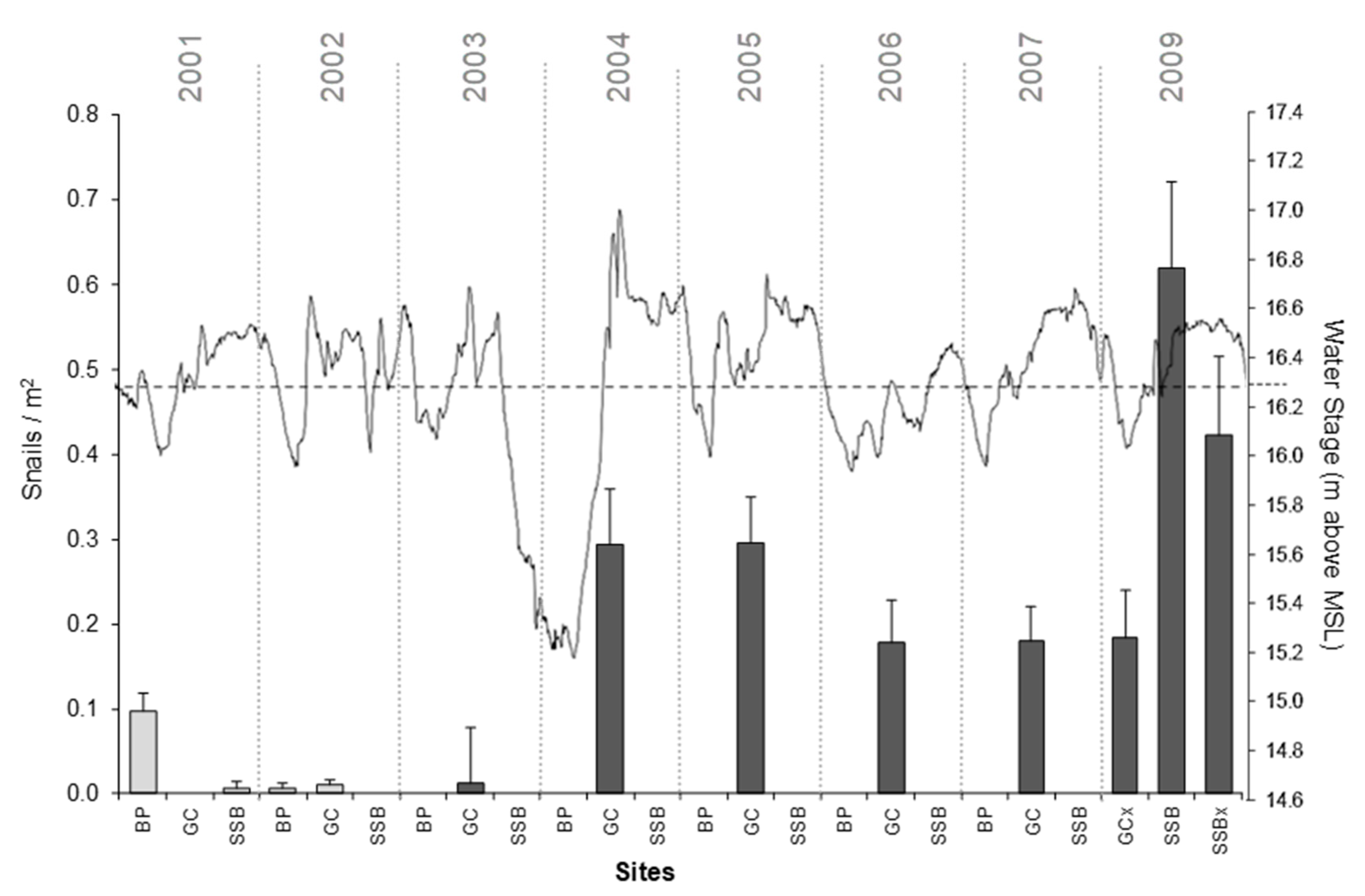
3.2. Chronology of Establishment of P. maculata in LTOHO
3.3. Chronology of Establishment of P. maculata in WCA3A
4. Discussion
4.1. Overview of P. maculata Establishment and Dispersal
4.2. P. maculata Dispersal in LTOHO
4.3. P. maculata Dispersal in WCA3A
4.4. Implications of P. maculata Range Expansion
4.5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey (USGS). Nonindigenous Aquatic Species Database. Available online: https://nas.er.usgs.gov/queries/SpeciesList.aspx?Group=Mollusks (accessed on 1 May 2019).
- Ricciardi, A. Ecology of invasive alien invertebrates. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. [Google Scholar]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Howells, R.G.; Burlakova, L.F.; Karatayev, A.Y.; Marfurt, R.K.; Burks, R.L. Native and introduced Ampullariidae in North America: History, status, and ecology. In Global Advances in the Ecology and Management of Golden Apple Snails; Joshi, R.C., Sebastian, L.S., Muñoz, N.E., Eds.; Philippine Rice Research Institute: Maligaya, Philippines, 2006; pp. 73–112. [Google Scholar]
- Ricciardi, A.; MacIsaac, H.J. Impacts of biological invasions on freshwater ecosystems. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 211–224. [Google Scholar]
- Kappes, H.; Haase, P. Slow, but steady: Dispersal of freshwater molluscs. Aquat. Sci. 2012, 74, 1–14. [Google Scholar] [CrossRef]
- Counts, C.L., III. The zoogeography and history of the invasion of the United States by Corbicula fluminea (Bivalvia: Corbiculidae). Am. Malacol. Bull. 1986, 2, 7–39. [Google Scholar]
- Zaranko, D.T.; Farara, D.G.; Thompson, F.G. Another exotic mollusc in the laurentian Great Lakes: The New Zealand native Potamopyrgus antipodarum (Gray 1843) (Gastropoda, Hydrobiidae). Can. J. Fish. Aquat. Sci. 1997, 54, 809–814. [Google Scholar] [CrossRef]
- Belz, C.E.; Darrigran, G.; Netto, O.S.M.; Boeger, W.A.; Ribeiro, P.J. Analysis of four dispersion vectors in inland waters: The case of the invading bivalves in South America. J. Shellfish Res. 2012, 31, 777–785. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Padilla, D.K.; Minchin, D.; Boltovskoy, D.; Burlakova, L.E. Changes in global economies and trade: The potential spread of exotic freshwater bivalves. Biol. Invasions 2007, 9, 161–180. [Google Scholar] [CrossRef]
- Keller, R.P.; Drake, J.M.; Lodge, D.M. Fecundity as a basis for risk assessment of nonindigenous freshwater molluscs. Conserv. Biol. 2007, 21, 191–200. [Google Scholar] [CrossRef]
- Tomiyama, K.; Nakane, M. Dispersal patterns of the giant African snail, Achatina fulica (Ferussac) (Stylommatophora: Achatinidae), equipped with a radio-transmitter. J. Molluscan Stud. 1993, 59, 315–322. [Google Scholar] [CrossRef]
- Wolfenbarger, D.O. Dispersion of the giant African snail Achatina fulica. Q. J. Fla. Acad. Sci. 1971, 34, 48–52. [Google Scholar]
- Raut, S.K.; Barker, G.M. Achatina fulica Bowdich and other Achatinidae as pests in tropical agriculture. In Molluscs as Crop Pests; Barker, G.M., Ed.; CAB International: Wallingford, UK, 2002; pp. 55–114. [Google Scholar]
- Cowie, R.H. Apple snails (Ampullariidae) as agricultural pests: Their biology, impacts, and management. In Molluscs as Crop Pests; Barker, G.M., Ed.; CAB International: Wallingford, UK, 2002; pp. 145–192. [Google Scholar]
- Horgan, F.G.; Stuart, A.M.; Kudavidanage, E.P. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica 2014, 54, 90–100. [Google Scholar] [CrossRef]
- Cowie, R.H.; Hayes, K.A. Apple snails. In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Earthscan: New York, NY, USA, 2012; pp. 207–221. [Google Scholar]
- Rawlings, T.A.; Hayes, K.A.; Cowie, R.H.; Collins, T.M. The identity, distribution, and impacts of non-native apple snails in the continental United States. BMC Evol. Biol. 2007, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Winterbourne, M.J. Population studies on the New Zealand freshwater gastropod, Potamoptrgus antipodarum (Gray). J. Molluscan Stud. 1970, 39, 139–149. [Google Scholar] [CrossRef]
- Lucy, F. Early life stages of Dreissena polymorpha (zebra mussel): The importance of long-term datasets in invasion ecology. Aquat. Invasions 2006, 1, 171–182. [Google Scholar] [CrossRef]
- Burky, K.A.; Burky, A.J. Buoyancy changes as related to respiratory behavior in an amphibious snail, Pomacea urceus (Muller), from Venezuela. Nautilus 1977, 91, 97–104. [Google Scholar]
- Valentine-Darby, P.L.; Darby, P.C.; Percival, H.F. Gender-based differences in Florida apple snail (Pomacea paludosa) movements. Malacologia 2011, 54, 109–118. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martín, P.R. A lentic dweller in lotic habitats: The behavior of the invasive South American apple snail Pomacea canaliculata in flowing water. Aquatic Ecol. 2012, 46, 129–142. [Google Scholar] [CrossRef]
- Van Leeuwen, C.H.; Huig, N.; van der Velde, G.; Van Alen, T.A.; Wagemaker, C.A.; Sherman, C.D.; Klaassen, M.; Figuerola, J. How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshw. Biol. 2013, 58, 88–99. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- Marzolf, N.; Golladay, S.; McCormick, P.; Covich, A. Inter- and intra-annual apple snail egg mass dynamics in a large southeastern U.S. reservoir. Hydrobiologia 2018, 811, 155–171. [Google Scholar] [CrossRef]
- Thompson, F.G. Freshwater Snails of Florida: A Manual for Identification; University of Florida Press: Gainesville, FL, USA, 2004; pp. 1–94. [Google Scholar]
- Robertson, S.M. Potential Threats of the Exotic Apple Snail Pomacea insularum to Aquatic Ecosystems in Georgia and Florida. Master’s Thesis, University of Georgia, Athens, GA, USA, 2012. [Google Scholar]
- Benson, A.J. Pomacea maculata Perry 1810. U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, Florida, USA. 2018. Available online: https://nas.er.usgs.gov/queries/FactSheet.aspx?SpeciesID=2633 (accessed on 23 May 2018).
- Cattau, C.E.; Fletcher, R.J.; Reichert, B.E.; Kitchens, W.M. Counteracting effects of a non-native prey on the demography of a native predator culminate in positive population growth. Ecol. Appl. 2016, 26, 1952–1968. [Google Scholar] [CrossRef]
- Darby, P.C.; Mellow, D.J.; Watford, M.L. Food handling difficulties for snail kites capturing non-native apple snails. Fla. Field Nat. 2007, 35, 79–85. [Google Scholar]
- Burks, R.L.; Bernatis, J.; Byers, J.E.; Carter, J.; Martin, C.W.; McDowell, W.G.; van Dyke, J. Identity, reproductive potential, distribution, ecology and management of invasive Pomacea maculata in the southern United States. In Biology and Management of Invasive Apple Snails; Joshi, R.C., Cowie, R.H., Sebastian, L.S., Eds.; Philippine Rice Research Institute: Maligaya, Philippines, 2017; pp. 293–333. [Google Scholar]
- Hayes, K.A.; Cowie, R.H.; Thiengo, S.C.; Strong, E.E. Comparing apples with apples: Clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zool. J. Linn. Soc. 2012, 166, 723–753. [Google Scholar] [CrossRef]
- Pomacea Project. Literature Review of Florida Apple Snails and Snail Kites, and Recommendations for Their Adaptive Management. Submitted to National Park Service, Everglades National Park, Florida; Pomacea Project, Inc.: Pensacola, FL, USA, 2013. [Google Scholar]
- Hoyer, M.V.; Bachmann, R.W.; Canfield, D.E., Jr. Lake management (muck removal) and hurricane impacts to the trophic state of Lake Tohopekaliga, Florida. Lake Reserv. Manag. 2008, 24, 57–68. [Google Scholar] [CrossRef]
- Brush, J.; De Sa, M.; Welch, Z.; Enloe, C.; Kitchens, W. Monitoring Floral and Faunal Succession Following Lake Enhancement in the Littoral Reaches of Lake Tohopekaliga; Florida Cooperative Fish and Wildlife Research Unit; University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- Welch, Z.C. Restoring Pattern without Process in Lake Restoration: A Large-Scale Littoral Habitat Enhancement Project on Lake Tohopekaliga, Florida. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2009. [Google Scholar]
- Light, S.S.; Dineen, J.W. Water control in the Everglades: A historical perspective. In Everglades: The Ecosystem and Its Restoration; Davis, S.M., Ogden, J.C., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1994; pp. 47–84. [Google Scholar]
- Karunaratne, L.B.; Darby, P.C.; Bennetts, R.E. The effects of wetland habitat structure on Florida apple snail density. Wetlands 2006, 26, 1143–1150. [Google Scholar] [CrossRef]
- Wight, B.R.; Darby, P.C.; Fujisaki, I. Quantifying edge effects on apple snails (Pomacea paludosa) and their eggs at the junction of two wetland habitat types. J. Molluscan Stud. 2017, 83, 351–359. [Google Scholar] [CrossRef]
- Bennetts, R.E.; Kitchens, W.M.; De Angelis, D.L. Recovery of the snail kite in Florida: Beyond a reductionist paradigm. Trans. N. Am. Wildl. Nat. Resour. Conf. 1998, 63, 486–501. [Google Scholar]
- Lodge, T.E. Freshwater marshes: Water, weather, and fire. In The Everglades Handbook: Understanding the Ecosystem, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 35–54. [Google Scholar]
- Darby, P.C.; Bennetts, R.E.; Percival, H.F. Dry down impacts on apple snail (Pomacea paludosa) demography: Implications for wetland water management. Wetlands 2008, 28, 204–214. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Everglades Depth Estimation Network (EDEN) for Support of Biological and Ecological Assessments. 2019. Available online: https://sofia.usgs.gov/eden/ (accessed on 22 September 2019).
- Darby, P.C.; Bennetts, R.E.; Karunaratne, L.B. Apple snail densities in habitats used by foraging snail kites. Fla. Field Nat. 2006, 34, 37–47. [Google Scholar]
- Darby, P.C.; Fujisaki, I.; Mellow, D.J. The effects of prey density on capture times and foraging success of course-hunting adult snail kites. Condor 2012, 114, 755–763. [Google Scholar] [CrossRef]
- Darby, P.C.; Croop, J.D.; Bennetts, R.E.; Valentine-Darby, P.L.; Kitchens, W.M. A comparison of sampling techniques for quantifying abundance of the Florida apple snail (Pomacea paludosa, Say). J. Molluscan Stud. 1999, 65, 195–208. [Google Scholar] [CrossRef]
- Bennetts, R.E.; Darby, P.C.; Karunaratne, L.B. Foraging patch selection by snail kites in response to vegetation structure and prey abundance and availability. Waterbirds 2006, 29, 88–94. [Google Scholar] [CrossRef]
- Darby, P.C.; Fujisaki, I.; Mellow, D.L.; Therrien, M. Hydrologic and legacy effects linked to apple snail population trends in the Everglades. (in preparation)
- SAS Inc. User’s Guide, Version 9.2, 2nd ed.; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Cattau, C.; Kitchens, W.; Reichert, B.; Olbert, J.; Pias, K.; Martin, J.; Zweig, C. Snail Kite Demography Annual Report 2009. Prepared for U.S. Army Corps of Engineers; Florida Cooperative Fish and Wildlife Research Unit; University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- South Florida Water Management District (SFWMD); Florida Fish and Wildlife Conservation Commission; Florida Department of Environmental Protection; Florida Department of Agriculture and Consumer Services; U.S. Army Corps of Engineers; U.S. Fish and Wildlife Service; Osceola County. 2011 Interagency Draft, Kissimmee Chain of Lakes Long-Term Management Plan; SFWMD: West Palm Beach, FL, USA, 2011.
- Darby, P.C.; Valentine-Darby, P.L.; Percival, H.F.; Kitchens, W.M. Florida apple snail responses to lake habitat restoration activity. Archiv für Hydrobiologie 2004, 161, 561–575. [Google Scholar] [CrossRef]
- Cattau, C.E.; Martin, J.; Kitchens, W.M. Effects of an exotic prey species on a native specialist: Example of the snail kite. Biol. Conserv. 2010, 143, 513–520. [Google Scholar] [CrossRef]
- Pias, K.E.; Fletcher, R.J.; Kitchens, W.M. Assessing the value of novel habitats to snail kites through foraging behavior and nest survival. J. Fish. Wildl. Manag. 2016, 7, 449–460. [Google Scholar] [CrossRef]
- Langeland, K. Hydrilla verticillata (L.F.) Royle (Hydrocharitaceae), “The Perfect Aquatic Weed”. Castanea 1996, 61, 293–304. [Google Scholar]
- Madeira, P.T.; Jacono, C.C.; Van, T.K. Monitoring hydrilla using two RAPD procedures and the nonindigenous aquatic species database. J. Aquat. Plant. Manag. 2000, 38, 33–40. [Google Scholar]
- South Florida Water Management District (SFWMD). Excerpt from Kissimmee Chain of Lakes (KCOL) Long-Term Management Plan (LTMP) Chapter 4: Management Objectives and Priorities; South Florida Water Management District (SFWMD): Palm Beach, FL, USA, 2009.
- Carlsson, N.O.L.; Bronmark, C.; Hansson, L. Invading herbivory: The golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 2004, 85, 1575–1580. [Google Scholar] [CrossRef]
- Joshi, R.C.; Sebastian, L.S.; Muñoz, N.E. Global Advances in the Ecology and Management of Golden Apple Snails; Philippine Rice Research Institute: Maligaya, Philippines, 2006. [Google Scholar]
- Halwart, M. The golden apple snail Pomacea canaliculata in Asian rice farming systems: Present impact and future threat. Int. J. Pest. Manag. 1994, 40, 199–206. [Google Scholar] [CrossRef]
- Boland, B.B.; Meerhoff, M.; Fosalba, C.; Mazzeo, N.; Barnes, M.A.; Burks, R.L. Juvenile snails, adult appetites: Contrasting resource consumption between two species of applesnails (Pomacea). J. Molluscan Stud. 2008, 74, 47–54. [Google Scholar] [CrossRef]
- Baker, P.; Zimmanck, F.; Baker, S.M. Feeding rates of an introduced freshwater gastropod Pomacea insularum on native and nonindigenous aquatic plants in Florida. J. Molluscan Stud. 2010, 76, 138–143. [Google Scholar] [CrossRef]
- Morrison, W.E.; Hay, M.E. Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: Invasives eat more and grow more. Biol. Invasions 2011, 13, 945–955. [Google Scholar] [CrossRef]
- Monette, D.; Ewe, S.; Dinkins, J.M.; Markwith, S.H. Interactions of exotic and native Pomacea with wetland vegetation structure in the Greater Everglades, Florida, USA. Fundam. Appl. Limnol. 2017, 189, 291–299. [Google Scholar] [CrossRef]
- Gettys, L.A.; Haller, W.T.; Mudge, C.R.; Koschnick, T.J. Effect of temperature and feeding preference on submerged plants by the island apple snail, Pomacea insularum (d’Orbigny, 1839) (Ampullariidae). Veliger 2008, 50, 248–254. [Google Scholar]
- Van Dyke, J. Lake Munson: A Case Study of the Impact of Exotic Apple Snails on Aquatic Vegetation. 2009. Available online: https://snailbusters.wordpress.com/2009/08/24/lake-munson-a-case-study-of-the-impact-of-exotic-apple-snails-on-aquatic-vegetation/ (accessed on 17 October 2017).
- Burlakova, L.E.; Karatayev, A.Y.; Padilla, D.K.; Cartwright, L.D.; Hollas, D.N. Wetland restoration and invasive species: Apple snail (Pomacea insularum) feeding on native and invasive aquatic plants. Restor. Ecol. 2009, 17, 433–440. [Google Scholar] [CrossRef]
- Cattau, C.E.; Darby, P.C.; Fletcher, R.J., Jr.; Kitchens, W.M. Reproductive response of the endangered snail kite to variation in prey density. J. Wildl. Manag. 2014, 78, 620–631. [Google Scholar] [CrossRef]
- Hayes, K.A.; Burks, R.L.; Castro-Vazquez, A.; Darby, P.C.; Heras, H.; Martín, P.R.; Qiu, J.; Thiengo, S.C.; Vega, I.A.; Wada, T.; et al. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 2015, 58, 245–303. [Google Scholar] [CrossRef]
- Cole, R.A.; Thomas, N.J.; Roderick, C.L. Bothrigaster variolaris (Trematoda: Cyclocoelidae) infection in two Florida snail kites (Rostrhamus sociabilis plumbeus). J. Wildl. Dis. 1995, 31, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.R.; Haynie, R.S.; Williams, S.M.; Wilde, S.B. Alternate food-chain transfer of the toxin linked to avian vacuolar myelinopathy and implications for the endangered Florida snail kite (Rostrhamus sociabilis). J. Wildl. Dis. 2016, 52, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.L.; Pomory, C.M.; Darby, P.C. Density effects of native and exotic snails on growth in juvenile apple snails Pomacea paludosa (Gastropoda: Ampullariidae): A laboratory experiment. J. Molluscan Stud. 2008, 74, 355–362. [Google Scholar] [CrossRef]
- Posch, H.; Garr, A.L.; Reynolds, E. The presence of an exotic snail, Pomacea maculata, inhibits growth of juvenile Florida apple snails, Pomacea paludosa. J. Molluscan Stud. 2013, 79, 383–385. [Google Scholar] [CrossRef]
- Bernatis, J.L.; Warren, G.L. Effectiveness of a hand removal program for management of nonindigenous apple snails in an urban pond. Southeast. Nat. 2014, 13, 607–618. [Google Scholar] [CrossRef]
- Martin, C.W.; Bayha, K.M.; Valentine, J.F. Establishment of the invasive island apple snail Pomacea insularum (Gastropoda: Ampullaridae) and eradication efforts in Mobile, Alabama, USA. Gulf Mex. Sci. 2012, 30, 30–38. [Google Scholar] [CrossRef]
- Green, A.J.; Figuerola, J. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Divers. Distrib. 2005, 11, 149–156. [Google Scholar] [CrossRef]
- Van Leeuwen, C.H.; van der Velde, G. Prerequisites for flying snails: External transport potential of aquatic snails by waterbirds. Freshw. Sci. 2012, 31, 963–972. [Google Scholar] [CrossRef]
- Cowie, R.H.; Robinson, D.G. Pathways of introduction of nonindigenous land and freshwater snails and slugs. In Invasive Species: Vectors and Management Strategies; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 93–121. [Google Scholar]
- Waterkeyn, A.; Vanschoenwinkel, B.; Elsen, S.; Anton-Pardo, M.; Grillas, P.; Brendonck, L. Unintentional dispersal of aquatic invertebrates via footwear and motor vehicles in a Mediterranean wetland area. Aquat. Conserv. 2010, 20, 580–587. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef]
- Darby, P.C.; DeAngelis, D.L.; Romanach, S.S.; Suir, K.; Bridevaux, J. Modeling apple snail population dynamics on the Everglades landscape. Landsc. Ecol. 2015, 30, 1497–1510. [Google Scholar] [CrossRef]




| % of Traps | |||||||
|---|---|---|---|---|---|---|---|
| Vegetation Type | Species | 2001–2003 | 2004–2007 | ||||
| GC* | BP | SSB | GC | BP | SSB | ||
| Oviposition | Pontederia cordata | 100 | 100 | 100 | 45 | 51 | 75 |
| Oviposition | Panicum repens | -- | -- | -- | 44 | 48 | 25 |
| Oviposition | Sagittaria lancifolia | -- | -- | -- | 5 | 1 | -- |
| Oviposition | Eleocharis cellulosa | -- | -- | -- | 5 | -- | -- |
| other | Alternanthera philoxeroides | 80 | -- | -- | 44 | 45 | 37 |
| other | Luziola fluitans | 4 | -- | 26 | 37 | 34 | 44 |
| other | Utricularia purpurea | 8 | 100 | 74 | 1 | 7 | 4 |
| other | Bacopa caroliniana | 8 | -- | -- | 6 | 1 | 1 |
| other | Hydrilla verticillata | -- | -- | -- | 1 | 9 | -- |
| other | Vallisneria americana | -- | -- | -- | 6 | -- | -- |
| other | Unknown grass | -- | -- | -- | 5 | 4 | 14 |
| Vegetation Type | Species | % of Traps | ||
|---|---|---|---|---|
| 2006–2010 | 2011–2015 Non-native only Sites | 2011–2015 Native only Sites | ||
| Oviposition | Eleocharis cellulosa | 76 | 74 | 79 |
| Oviposition | Panicum hemitomon | 23 | 5 | 7 |
| Oviposition | Paspalidium geminatum | 1 | 14 | 14 |
| Oviposition | Bacopa caroliniana | 38 | 35 | 62 |
| other | Chara sp. | 24 | 20 | 5 |
| other | Potamogeton sp. | 15 | 15 | 5 |
| other | Utricularia purpurea | 18 | 27 | 28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierre, S.M.M.; Darby, P.C.; Valentine-Darby, P.L.; Mellow, D.J.; Therrien, M.; Watford, M. Contrasting Patterns of Pomacea maculata Establishment and Dispersal in an Everglades Wetland Unit and a Central Florida Lake. Diversity 2019, 11, 183. https://doi.org/10.3390/d11100183
Gutierre SMM, Darby PC, Valentine-Darby PL, Mellow DJ, Therrien M, Watford M. Contrasting Patterns of Pomacea maculata Establishment and Dispersal in an Everglades Wetland Unit and a Central Florida Lake. Diversity. 2019; 11(10):183. https://doi.org/10.3390/d11100183
Chicago/Turabian StyleGutierre, Silvia M. M., Philip C. Darby, Patricia L. Valentine-Darby, David J. Mellow, Michel Therrien, and Miranda Watford. 2019. "Contrasting Patterns of Pomacea maculata Establishment and Dispersal in an Everglades Wetland Unit and a Central Florida Lake" Diversity 11, no. 10: 183. https://doi.org/10.3390/d11100183




