Abstract
Climate change and the overall increase of seawater temperature are causing a poleward shift in species distribution, which includes a phenomenon described as the tropicalization of temperate regions. This work aims to report the first records of four species off the southwestern Iberian Peninsula, namely, the oceanic puffer Lagocephalus lagocephalus (Linnaeus, 1758), the Madeira rockfish Scorpaena maderensis Valenciennes, 1833, the ornate wrasse Thalassoma pavo (Linnaeus, 1758), and the bearded fireworm Hermodice carunculata (Pallas, 1766). These last three species, along with other occurrences of aquatic fauna and flora along the Portuguese coast, reveal an ongoing process of poleward expansion of several species, which urgently necessitates a comprehensive survey along the entire Iberian Peninsula. The putative origins of these subtropical and tropical species off continental Portugal are discussed, as well as the potential public health problems that two of the four reported species may cause.
1. Introduction
The effects of climate change are becoming evident across oceans, through changes of sea level, ocean primary productivity, ocean acidification, water temperature, and shifts in species distribution ranges [1]. In some temperate regions, the increase in seawater temperature is causing a poleward movement of species—a phenomenon known as the tropicalization of temperate regions [2]. The global trend is that this tropicalization phenomenon is occurring in many marine ecosystems [3]. Along the west and east coasts of Australia, dense kelp forests are disappearing, with records of 100 km range contractions on the west coast which are then followed by increases in the populations of subtropical and tropical herbivorous fish that suppress kelp recovery [4,5,6]. Other important habitat-forming species, such as coral reefs, have been expanding their poleward distribution along the Japanese coast at a rate of up to 14 km/year [7]. Simultaneously, tropical fish species are settling in these regions and being able to withstand the colder winter waters off Japan [8]. In the northern region of the Gulf of Mexico, an increasing number of diverse southern tropical species (e.g., fish, manatees, turtles, warm-water corals, black mangroves) are reshaping the characteristic seagrass meadows [9]. Similar cases have been observed in the northern Mediterranean Sea since the early 1990s, where several southern warmer-water fish species have been recorded [2,10,11].
Along the southwestern and western coasts of the Iberian Peninsula (Europe), the distribution range of several algae species shifted northwards to the order of hundreds and thousands of kilometres [12,13]. There are also examples of new species being reported off southern Portugal, such as the brown mussel Perna perna (Linnaeus, 1758) [14] and the parrotfish Sparisoma cretense (Linnaeus, 1758) [15], which suggests that a wide array of species are shifting their northern distribution limit from northern Africa to the Atlantic coast of the Iberian Peninsula.
In this context, this work aims at reporting four new accounts of subtropical/tropical faunal species observed for the first time off the south coast of Portugal and in the Guadiana estuary (SE Portugal/SW Spain). These new records for the southwestern Iberian Peninsula include three Teleostei, i.e., the Madeira rockfish Scorpaena maderensis Valenciennes, 1833, the ornate wrasse Thalassoma pavo (Linnaeus, 1758), and the oceanic puffer Lagocephalus lagocephalus (Linnaeus, 1758), as well as a polychaete annelid, the bearded fireworm Hermodice carunculata (Pallas, 1766).
2. Materials and Methods
2.1. Study Area
Three out of the four new records of marine fauna reported in this work were registered off Portimão (SW Portugal) (Figure 1A), at one natural rocky reef and two artificial reefs. The natural rocky reef is known as “Pedra do Mariano” (37°06.143’ N, 8°34.610’ W), and the reef wall lies between 14 and 17 meters deep, surrounded mostly by rocky bottoms and scattered sandy areas (Figure 1B). The artificial reefs are two scuttled ships (Figure 1B): the “Oliveira e Carmo” corvette (37°05.501’ N 8°34.964’ W) and the “Almeida Carvalho” hydrographic ship (37°05.391’ N, 8°34.960’ W), which were intentionally sunk to form the Ocean Revival underwater park (www.oceanrevival.org) on 30 October 2012 and 21 September 2013, respectively. These ships lie at a depth of 30 meters on sandy bottoms and are elevated to a minimum depth between 14 and 18 meters. A fourth species was registered in the lower Guadiana estuary (SE Portugal/SW Spain) off Castro Marim (37°12.717’ N, 7°24.867’ W) (Figure 1C).
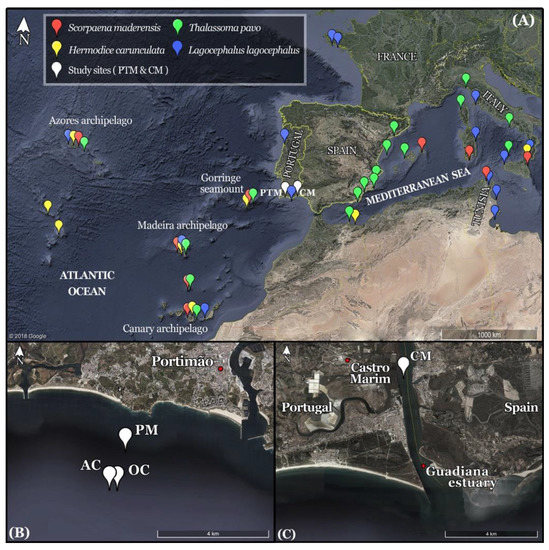
Figure 1.
(A) The location of the two main study sites off Portimão (PTM) and in the lower Guadiana estuary off Castro Marim (CM) (SE Portugal/SW Spain) is shown in the context of the Iberian Peninsula and northern African coast. The location of the Gorringe seamount and of the three Macaronesian archipelagos is also signled. The markers on the Macaronesian islands only reflect the presence of each species at each archipelago and not their precise distribution around each island. The species distribution was compiled on the basis of several scientific publications. (B) Location of the study sites off Portimão (PM, Pedra do Mariano; OC, Oliveira e Carmo corvette; AC, Almeida Carvalho hydrographic ship) and (C) in the lower Guadiana estuary off Castro Marim (CM). Maps retrieved from Google Earth.
2.2. Data Collection
In the region off Portimão, observations were made during non-systematic recreational SCUBA dives at the three sites, which occurred between 9 am and 11 am. Specimens were photographed with a Canon G16 digital camera (12 megapixel resolution). The depth at which species were photographed was annotated, as were their size and sex whenever possible. Size was estimated by including an object in the photograph to be used for scale, while sex was determined based on the sexual dimorphism characteristics if the species displayed such traits.
In the Guadiana estuary, a local fisherman collected two specimens with a longline gear deployed overnight, but only one specimen was delivered to us for identification. In the laboratory, this specimen was identified and sexed, its total fresh weight (±1 g) was determined, several morphometric and meristic characteristics were measured and counted, and the stomach content analyzed.
The identification of species was based on the photographs taken and on the collected specimen, following the identification books of Saldanha [16] and Louisy [17].
3. Results
During the non-systematic diving surveys done off Portimão, three species were identified as new additions to the southwestern Iberian Peninsula’s fauna. These species were the Madeira rockfish S. maderensis Valenciennes, 1833, the ornate wrasse T. pavo (Linnaeus, 1758), and the bearded fireworm H. carunculata (Pallas, 1766), which are characteristic of subtropical and tropical regions.
The first Madeira rockfish specimen was recorded on 29 July 2016, in the “Oliveira e Carmo” corvette at a depth of 24 meters (Figure 2A). A second individual was observed in the same location on October 3, 2016, at a depth of 20 meters (Figure 2B).
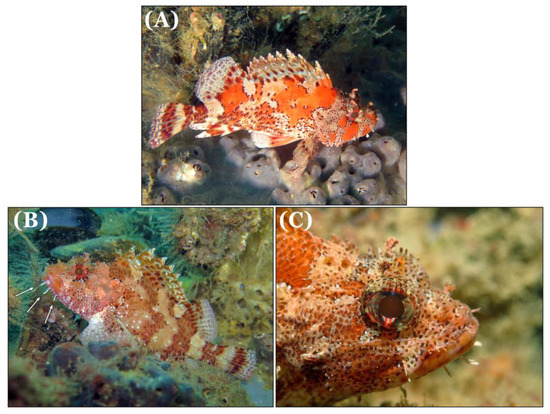
Figure 2.
Specimens of Madeira rockfish Scorpaena maderensis Valenciennes, 1833 observed on July 29, 2016 (A) and on October 3, 2016 (B), both on the “Oliveira e Carmo” corvette, and on March 16, 2019, on the “Almeida Carvalho” hydrographic ship (C). Arrows indicate the distinctive long white tentacles in the lower mandible (B). Photographs taken by João Encarnação.
The first observed ornate wrasse was a female and was observed on May 8, 2017, in the “Almeida Carvalho” hydrographic ship at a depth of 17 meters (Figure 3A). A second specimen was observed in the “Oliveira e Carmo” on May 15, 2017 (no photograph taken). A third specimen was observed in the “Almeida Carvalho” on May 31, 2017, at a depth of 16 meters (Figure 3B). This individual’s coloration pattern suggests that it was going through the process of changing sex, from female to male. A fourth ornate wrasse was a female, observed in the same location on September 19, 2018, at a depth of 16 meters (Figure 3C). A fifth individual was observed in the “Oliveira e Carmo” on September 22, 2018, at a depth of 20 meters (Figure 3D). This individual was in an advance transitional phase from female to male. On October 3, 2018, two other individuals (male and female) were identified in the “Oliveira e Carmo” at a depth of 17–20 meters (Figure 3E).

Figure 3.
Specimens of ornate wrasse Thalassoma pavo (Linnaeus, 1758) observed on May 8, 2017 (A), May 31, 2017 (B), September 19, 2018 (C), and March 16, 2019 (F), on the “Almeida Carvalho” hydrographic ship. Other specimens were observed on the “Oliveira e Carmo” corvette on September 22, 2018 (D) and on October 3, 2018 (E, left: male, right: female). Photographs taken by João Encarnação.
The first and only record of a bearded fireworm occurred on September 7, 2018, at the “Pedra do Mariano” rocky reef at a depth of 16 meters. The total length of this specimen was circa 33 cm (Figure 4).

Figure 4.
The bearded fireworm Hermodice carunculata (Pallas, 1766) observed on 7 September 2018, at the “Pedra do Mariano” rocky reef. The approximate total length was 33 cm. Photographs taken by João Encarnação.
In the Guadiana estuary, two specimens of oceanic puffer L. lagocephalus (Linnaeus, 1758) were collected by local fishermen, the first on May 23, 2017, and the second on October 17, 2017. However, only the latter specimen was delivered to us for analysis. This specimen was a male, with a total length of 37.2 cm and total fresh weight of 453 g (Figure 5). All the morphometric and meristic characteristics are described in Table 1. The stomach was empty.

Figure 5.
Male oceanic puffer Lagocephalus lagocephalus (Linnaeus, 1758) specimen collected on October 17, 2017, in the lower Guadiana estuary off Castro Marim. Photographs taken by Vânia Baptista.

Table 1.
Morphometric and meristic characteristics, as well as total fresh weight of the male oceanic puffer L. lagocephalus (Linnaeus, 1758) specimen collected in the lower Guadiana estuary off Castro Marim (37°12’43” N, 7°24’52” W) on 17 October 2017.
4. Discussion
This work provides the first account of four faunal species present off the Algarve (southwestern Iberian Peninsula, Europe). Of these, the Madeira rockfish, the ornate wrasse, and the bearded fireworm are typically from the subtropical Macaronesia archipelagos [18,19,20] (Figure 1A). This fact might illustrate the influence of the rising seawater temperature on the poleward expansion of subtropical or tropical species, i.e., tropicalization.
The Algarve coast is located in the south European Atlantic shelf ecoregion which borders three other ecoregions: the Macaronesia archipelagos, the northwestern African coast, and the Mediterranean Sea [21]. The Algarve’s location—at the intersection of three other ecoregions— increases the possibility of the appearance of species that are typically from other ecoregions.
The Madeira rockfish is a common benthic species in all the Macaronesian archipelagos and along the coasts of Morocco, Mauritania, and Senegal [22]. It is also present in the Mediterranean Sea, from Greek waters [23] to the central Mediterranean [24,25], and eastwards to the Balearic Islands [26]. The colonization of the Balearic Islands by the Madeira rockfish probably happened in the beginning of the 1990s as a result of a northward expansion due to increasing water temperatures in the southern Mediterranean [26]. However, misidentifications might have hindered the report of this species earlier, as it occurred with Scorpaena porcus Linnaeus, 1758 in the Mediterranean Sea [24], a species that is also present off southern Portugal along with the more common Scorpaena notata Rafinesque, 1810 [27].
Regarding the ornate wrasse, this species was always abundant in the eastern Mediterranean Sea [28], but its presence in the northwestern Mediterranean was sporadic until 1988. During the early 1990s, ornate wrasse density more than doubled, and juveniles were accounted for in subsequent years [10,28]. By 1997, a self-sustained population existed in the Ligurian Sea, with juveniles and adult males and females exhibiting mating behaviours [29]. Like other Labridae, the ornate wrasse is a protogynous hermaphrodite, which may explain the occurrence of mostly females in the first three records in the southwestern Iberian Peninsula. These hermaphroditic females can, given the right conditions, become successful territorial males, as shown by other Thalassoma species [30]. So, we propose that the occurrence of transition-phase individuals and terminal-phase males during late 2018 (Figure 3D,E) has the potential to establish them successfully off the southwestern Iberian Peninsula.
The bearded fireworm is widely distributed in the Atlantic Ocean from the Caribbean to the Macaronesian islands, as well as in the Mediterranean and Red seas [20]. It is a voracious and generalist omnivorous species, preying mainly on anemones, gorgonians, and milleporid hydrocorals [31,32]. This species may pose public health risks if it becomes abundant off the Algarve coast, since its calcareous chaetae induce intense pain, inflammation, and edema when they come into contact with human skin. It is important to mention that there is no agreement on classifying this species as venomous, poisonous, or toxic [33]. Nevertheless, precautionary measures should be implemented to prevent people from touching this species if it becomes widespread, especially in highly tourist regions such as the Algarve.
The area of origin of these three new taxa is difficult to pinpoint, but the Macaronesian area emerges as the likely origin. The Madeira and Canary Islands are located circa 800 and 950 kilometres southwest of the Algarve, respectively, and the Azores Islands circa 1450 kilometers westward of Cape Saint Vincent (Figure 1A). Furthermore, the location of the Gorringe Seamount is circa 270 km west-southwest of Cape Saint Vincent and may function as a stepping-stone to facilitate the arrival of Macaronesian species, as suggested in the case of the damselfish Abudefduf luridus (Cuvier, 1830) [34]. Indeed, all three of these species are present in the Gorringe Seamount (Figure 1A): the Madeira rockfish was first recorded in 2006 [35], the ornate wrasse in 1998 [34], and the bearded fireworm in 2005 [36]. Apart from the swimming capabilities of the fish species, they can also tolerate cold water temperatures, since we observed the Madeira rockfish and the ornate wrasse specimens when the water was at 15 °C (March 2019), similarly to observations made in the northwestern Mediterranean [37]. These species also display reproductive strategies that enable them to disperse given the right oceanographic conditions, specifically if they are able to use branches of the Azores Current which extends into the Gulf of Cadiz [38,39].
Although the reproductive biology of the Madeira rockfish remains unknown, it is possible to make comparisons with similar rockfish species, namely, with S. notata, a common species along the Algarve coast [27]. The females of this rockfish species deposit between 6000 and 33,000 eggs into a gelatinous matrix that provides mechanical protection while also acting as a floatation mechanism [40,41], which thus increases their dispersal potential. In the case of the ornate wrasse, spawning is pelagic, and the larval phase lasts between 38 and 49 days, which is considered to be long in comparison to other Labridae [42]. The reproductive biology of the bearded fireworm is still poorly known. Nevertheless, the genus Hermodice is presumed to be able to reproduce both sexually through the release of planktonic gametes and asexually, although this has never been demonstrated [43]. The long-lived planktotrophic larval phase increases the species potential to colonize new habitats [44]. We would like to emphasize that H. carunculata is considered the valid nomenclature and that Hermodice nigrolineata is considered a synonym [43], though this is controversial (Godfried Van Moorsel, Joachim Langeneck, Peter Wirtz, personal communications). Therefore, broader geographic-scale studies must be conducted to resolve the genus taxonomy and validate the identification of Portimão’s Hermodice.
The case of the oceanic puffer should be approached more carefully because the presence of oceanic puffers away from tropical and subtropical regions is often reported [45,46,47,48,49,50,51,52,53], including in estuarine ecosystems [50,54]. However, the report of oceanic puffers in the Guadiana came as a surprise to local fishers. Still, this new account cannot be linked with climate change, since the oceanic puffer was reported further north off Great Britain in the late 1940s [45,46] and 1960s [47] and in the North Sea in the 1970s [55], i.e., during a period when the impact of climate change was negligible [56]. In the Mediterranean Sea, and as far as we are aware, the first record dates to 1878 [53]. So, the increased frequency of reports in the Mediterranean Sea and in the northeastern Atlantic may simply reflect the increased research efforts of current times [48,49,51,52,53,57,58,59] However, all these facts do not imply that climate change is not inducing, or will not induce, a poleward range expansion of oceanic puffer populations. In the case of the Guadiana estuary, both climate change and the disruption of natural river flow caused by the Alqueva dam might have contributed to the presence of oceanic puffer specimens in the estuary in tandem with the proximity of the Algarve to subtropical regions. Overall, the oceanic puffer is not a good example to illustrate the impact of climate change on aquatic species redistribution, at least for now. Similar to the bearded fireworm, the toxicity of the ocean puffer may induce public health problems [53], which discourages their consumption [58,60]. So, scientists must continue their efforts to document the aquatic fish fauna, while public health authorities should act promptly if the abundance of oceanic puffer increases in the future.
Other faunal species with subtropical or tropical origins have already been identified off the Algarve, such as the parrotfish S. cretense (Linnaeus, 1758) in the Ria Formosa lagoon [15], the hermit crab Calcinus tubularis (Linnaeus, 1767) in shallow rocky reefs [61], and the brown mussel P. perna (Linnaeus, 1758) in rocky intertidal shores [14]. However, examples are not restricted to animals. Indeed, there are many algae species expanding their distribution range, and others whose distribution range is retracting. Along the Portuguese coast, eight warm-water species have shown a poleward expansion between 59 km (Codium adhaerens C. Agard 1822) and 593 km (Sargassum flavifolium Kützing 1849). On the other hand, six cold-water species have exhibited a poleward retraction of their distribution between 62 km (Dumontia contorta (S.G.Gmelin) Ruprecht 1850) and 358 km (Palmaria palmata (Linnaeus) F.Weber & D.Mohr 1850) [12]. The case of the bladder wrack brown algae Fucus vesiculosus Linnaeus 1753 is particularly remarkable. Nowadays, F. vesiculosus is almost restricted to the northwestern Iberian Peninsula coast, with the southern distribution limit located in the Tagus estuary, while in 1986 it was present off the Moroccan coast, representing a 1250 km poleward shift in its distribution [13].
Finally, the collaboration of scientists with local fishers and the general public must be encouraged to anticipate the detection of new species, as has already occurred for the oceanic puffer (this study), the weakfish Cynoscion regalis (Bloch and Schneider, 1801), and the Atlantic blue crab Callinectes sapidus Rathbun, 1896 [62,63,64]. These experiences perfectly illustrate the importance of citizen-science initiatives in the advance of Marine Ecology.
5. Conclusions
The presence of the Madeira rockfish, ornate wrasse, and bearded fireworm off the southwestern Iberian Peninsula, and the possible presence of the oceanic puffer in the Guadiana estuary may represent examples of poleward-distribution range expansion of subtropical/tropical species. This hypothesis is supported by multiple lines of evidence from other species, as described in this paper. We advocate for the implementation of systematic surveys along the Atlantic coast of the Iberian Peninsula concomitantly with genetic analyses to test the tropicalization hypothesis. We also propose that these new fish species should be monitored and included in local fisheries management plans if their abundance increases.
Author Contributions
Conceptualization: J.E., M.A.T., and P.M. Collection of data: J.E., V.B., and J.C. Writing: J.E., P.M., and M.A.T. Revision: J.E, P.M., V.B., J.C., and M.A.T.
Funding
J.E. (SFRH/BD/140556/2018) and V.B. (SFRH/BD/104209/2014) have Ph.D. scholarships funded by Foundation for Science and Technology (FCT). P.M. has a scholarship funded by the Delta Stewardship Council and Delta Science Program under Grant No. 1167. The contents of this article do not necessarily reflect the views and policies of the Delta Stewardship Council, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. M.A.T. was funded by FCT, through the project UID/Multi/04326/2013, and by the European Regional Development Fund (COMPETE Program, Operational Competitiveness Program).
Acknowledgments
We would like to acknowledge the Subnauta diving center for assistance with logistics in Portimão and Mr. Antero Fernandes for reporting to us the capture of another new species in the Guadiana estuary. Two anonymous reviewers made important suggestions to improve the initial version of this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Morri, C. Global sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeographia 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef] [PubMed]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; de Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Perello, S.; Wernberg, T.; Langlois, T.J.; Vanderklift, M.A. Tropicalization strengthens consumer pressure on habitat forming seaweeds. Sci. Rep. 2017, 7, 820. [Google Scholar] [CrossRef] [PubMed]
- Yamano, H.; Sugihara, K.; Nomura, K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys. Res. Lett. 2011, 38, L04601. [Google Scholar] [CrossRef]
- Nakamura, Y.; Feary, D.A.; Kanda, M.; Yamaoka, K. Tropical fishes dominate temperate reef fish communities within Western Japan. PLoS ONE 2013, 8, e81107. [Google Scholar] [CrossRef] [PubMed]
- Heck, K.L., Jr.; Fodrie, F.J.; Madsen, S.; Baillie, C.J.; Byron, D.A. Seagrass consumption by native and a tropically associated fish species: Potential impacts of the tropicalization of the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2015, 520, 165–173. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Southern species in the Ligurian Sea (northern Mediterranean): New records and a review. Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 1994, 58/59, 181–197. [Google Scholar]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. U. K. 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Lima, F.P.; Ribeiro, P.A.; Queiroz, N.; Hawkins, S.J.; Santos, A.M. Do distributional shifts of northern and southern species of algae match the warming pattern? Glob. Chang. Biol. 2007, 13, 2592–2604. [Google Scholar] [CrossRef]
- Nicastro, K.R.; Zardi, G.I.; Teixeira, S.; Neiva, J.; Serrao, E.A.; Pearson, G.A. Shift happens: Trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.R.; Nicastro, K.R.; Serrão, E.A.; Zardi, G.I. First record of the brown mussel (Perna perna) from the European Atlantic coast. Mar. Biodivers. Rec. 2012, 5, e39. [Google Scholar] [CrossRef]
- Abecasis, D.; Bentes, L.; Ribeiro, J.; Machado, D.; Oliveira, F.; Veiga, P.; Gonçalves, J.M.S.; Erzini, K. First record of the Mediterranean parrotfish, Sparisoma cretense in Ria Formosa (south Portugal). Mar. Biodivers. Rec. 2008, 1, e27. [Google Scholar] [CrossRef]
- Saldanha, L. Fauna Submarina Atlântica, 4th ed.; Publicações Europa-América: Lisboa, Portugal, 1995; 361p. [Google Scholar]
- Louisy, P. Europe and Mediterranean Marine Fish Identification Guide; Ulmer: Paris, France, 2015; 512p. [Google Scholar]
- Harmelin-Vivien, M.L.; Harmelin, G.; Almeida, A.J. Structure of fish assemblages on coastal rocky shores of the Azores. Boletim do Museu Municipal do Funchal 2001, 6, 127–138. [Google Scholar]
- Tuya, F.; Boyra, A.; Sanchez-Jerez, P.; Barbera, C.; Haroun, R.J. Relationships between rocky-reef fish assemblages, the sea urchin Diadema antillarum and macroalgae throughout the Canarian Archipelago. Mar. Ecol. Prog. Ser. 2004, 278, 157–169. [Google Scholar] [CrossRef]
- Yáñez-Rivera, B.; Salazar-Vallejo, S.I. Revision of Hermodice Kinberg, 1857 (Polychaeta: Amphinomidae). Sci. Mar. 2011, 75, 251–262. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, A.Z.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Hureau, J.C.; Litvinenko, N.I. Scorpaenidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1211–1229. [Google Scholar]
- Ahnelt, V.H. Erstnachweis von Scorpaena maderensis Val., 1833 (Pisces, Scorpaenidae) für Kreta und Rhodos (Griechenland) mit Angaben zum bisher einzigen Fund dieser Art aus der Adria. Annalen des Naturhistorischen Museums in Wien 1983, 85B, 9–11. [Google Scholar]
- La Mesa, M.; La Mesa, G.; Micalizzi, M. Age and growth of Madeira scorpionfish, Scorpaena maderensis Valenciennes, 1833, in the central Mediterranean. Fish. Res. 2005, 74, 265–272. [Google Scholar] [CrossRef]
- Falzon, M.A. First records of Scorpaena maderensis (Pisces Scorpaeniformes Scorpaenidae) in inshore Maltese waters. Naturalista Siciliano 2011, 35, 419–423. [Google Scholar]
- Cardona, L.; Elices, M. Datos sobre la presencia en el litoral de Menorca (islas Baleares, Mediterráneo Occidental) de Parablennius pilicornis (Cuvier, 1829) y Scorpaena maderensis Valenciennes 1833. Bolletí de la Societat d’Història Natural de les Balears 2000, 43, 33–38. [Google Scholar]
- Leitão, F.; Baptista, V.; Zeller, D.; Erzini, K. Reconstructed catches and trends for mainland Portugal fisheries between 1938 and 2009: Implications for sustainability, domestic fish supply and imports. Fish. Res. 2014, 155, 33–50. [Google Scholar] [CrossRef]
- Francour, P.; Boudouresque, C.F.; Harmelin, J.G.; Harmelin-Vivien, M.L.; Quignard, J.P. Are the Mediterranean waters becoming warmer? Information from biological indicators. Mar. Pollut. Bull. 1994, 28, 523–526. [Google Scholar] [CrossRef]
- Vacchi, M.; Morri, C.; Modena, M.; La Mesa, G.; Bianchi, C.N. Temperature changes and warm-water species in the Ligurian Sea: The case of the ornate wrasse Thalassoma pavo (Linnaeus, 1758). Archo Oceanogr. Limnol. 2001, 22, 149–154. [Google Scholar]
- Warner, R.R.; Hoffman, S. Local population size as a determinant of mating system and sexual composition in two tropical marine fishes (Thalassoma spp.). Evolution 1980, 34, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Witman, J.D. Effects of predation by the fireworm Hermodice carunculata on milleporid hydrocorals. Bull. Mar. Sci. 1988, 42, 446–458. [Google Scholar]
- Chatzigeorgiou, G.; Sarropoulou, E.; Vasileiadou, K.; Brown, C.; Faulwetter, S.; Kotoulas, G.; Arvanitidis, C.D. Community structure and population genetics of Eastern Mediterranean polychaetes. Front. Mar. Sci. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Verdes, A.; Simpson, D.; Holford, M. Are Fireworms Venomous? Evidence for the Convergent Evolution of Toxin Homologs in Three Species of Fireworms (Annelida, Amphinomidae). Genome Biol. Evol. 2018, 10, 249–268. [Google Scholar] [CrossRef]
- Gonçalves, J.M.S.; Bispo, J.; Silva, J. Underwater survey of ichthyofauna of eastern Atlantic seamounts: Gettysburg and Ormonde (Gorringe Bank). Arch. Fish. Mar. Res. 2004, 51, 233–240. [Google Scholar]
- Abecasis, D.; Cardigos, F.; Almada, F.; Gonçalves, J.M.S. New records on the ichthyofauna of the Gorringe seamount (Northeastern Atlantic). Mar. Biol. Res. 2009, 5, 605–611. [Google Scholar] [CrossRef]
- OCEANA. The Seamounts of the Gorringe Bank; Fondazione Ermenegildo Zegna: Trivero, Italy, 2005; 72p. [Google Scholar]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Barton, E.D. Encyclopedia of Ocean Sciences; Steele, J.H., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: Florence, Italy, 2001; pp. 380–389. [Google Scholar]
- Pingree, R. Ocean structure and climate (Eastern North Atlantic): In situ measurement and remote sensing (altimeter). J. Mar. Biol. Assoc. U. K. 2002, 82, 681–707. [Google Scholar] [CrossRef]
- Muñoz, M.; Casadevall, M.; Bonet, S. The ovarian morphology of Scorpaena notata shows a specialized mode of oviparity. J. Fish Biol. 2002, 61, 877–887. [Google Scholar] [CrossRef]
- Muñoz, M.; Sàbat, M.; Vila, S.; Casadevall, M. Annual reproductive cycle and fecundity of Scorpaena notata (Teleostei: Scorpaenidae). Sci. Mar. 2005, 69, 555–562. [Google Scholar] [CrossRef]
- Raventós, N.; Macpherson, E. Planktonic larval duration and settlement marks on the otoliths of Mediterranean littoral fishes. Mar. Biol. 2001, 138, 1115–1120. [Google Scholar]
- Ahrens, J.; Borda, E.; Barroso, R.; Paiva, P.C.; Campbell, A.M.; Wolf, A.; Nugues, M.M.; Rouse, G.W.; Schulze, A. The curious case of Hermodice carunculata (Annelida: Amphinomidae): Evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basins. Mol. Ecol. 2013, 22, 2280–2291. [Google Scholar] [CrossRef]
- Schulze, A.; Grimes, C.J.; Rudek, T.E. Tough, armed and omnivorous: Hermodice carunculata (Annelida: Amphinomidae) is prepared for ecological challenges. J. Mar. Biol. Assoc. U. K. 2017, 97, 1075–1080. [Google Scholar] [CrossRef]
- Matheson, C. Records of the Globefish and Filefish. Nature 1950, 165, 193. [Google Scholar] [CrossRef]
- Went, A.E.J. Recent Irish records of rare fish. Nature 1950, 165, 1025. [Google Scholar] [CrossRef]
- Wheeler, A.; Blacker, R.W. Rare and little-known fishes in British seas in 1968 and 1969. J. Fish Biol. 1972, 4, 141–170. [Google Scholar] [CrossRef]
- Erzini, K.; Monteiro, P.; Araújo, A.; Castro, M. Limited mid-water scavenging of trawl discards. J. Mar. Biol. Assoc. U. K. 2003, 83, 731–734. [Google Scholar] [CrossRef]
- Dulčić, J.; Pallaoro, A. First record of the oceanic puffer (Lagocephalus lagocephalus lagocephalus Linnaeus, 1758), for the Adriatic Sea. J. Appl. Ichthyol. 2006, 22, 94–95. [Google Scholar] [CrossRef]
- Hardy, G.; Jing, L.; Leis, J.L.; Liu, M.; Matsuura, K.; Shao, K. Lagocephalus lagocephalus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2014; e.T190464A19929421. [Google Scholar]
- Farrag, M.; El-Haweet, A.A.K.; Akel, E.-S.k.A.; Moustafa, M.A. Occurrence of puffer fishes (Tetraodontidae) in the eastern Mediterranean, Egyptian coast—filling in the gap. BioInvasions Rec. 2016, 5, 47–54. [Google Scholar] [CrossRef]
- Erguden, D.; Gurlek, M.; Turan, C. First occurrence of the oceanic puffer, Lagocephalus lagocephalus (Linnaeus, 1758) in Iskenderun Bay, north-eastern Mediterranean, Turkey. J. Appl. Ichthyol. 2017, 33, 801–803. [Google Scholar] [CrossRef]
- Guardone, L.; Gasperetti, L.; Maneschi, A.; Ricci, E.; Susini, F.; Guidi, A.; Armani, A. Toxic invasive pufferfish (Tetraodontidae family) along Italian coasts: Assessment of an emerging public health risk. Food Control 2018, 91, 330–338. [Google Scholar] [CrossRef]
- Pombo, L.; Elliott, M.; Rebelo, J.E. Changes in the fish fauna of the Ria de Aveiro estuarine lagoon (Portugal) during the twentieth century. J. Fish Biol. 2002, 61, 167–181. [Google Scholar] [CrossRef]
- Yang, J. The dominant fish fauna in the North Sea and its determination. J. Fish Biol. 1982, 20, 635–643. [Google Scholar]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Quéro, P.; Bellail, R.; Casamajor, M.; Leaute, J.; Morandeau, G.; Morinière, P.; Spitz, J.; Vayne, J. Observations ichtyologiques effectuées en 2004. Ann. Soc. Sci. Nat. Charente-Maritime 2005, 9, 483–490. [Google Scholar]
- Saoudi, M.; Abdelmouleh, A.; Kammoun, W.; Ellouze, F.; Jamoussi, K.; El Feki, A. Toxicity assessment of the puffer fish Lagocephalus lagocephalus from the Tunisian coast. C. R. Biol. 2008, 331, 611–616. [Google Scholar] [CrossRef]
- Tsiamis, K.; Aydogan, O.; Bailly, N.; Balistreri, P.; Bariche, M.; Carden-Noad, S.; Corsini-Foka, M.; Crocetta, F.; Davidov, B.; Dimitriadis, C.; et al. New Mediterranean biodiversity records (July 2015). Mediterr. Mar. Sci. 2015, 16, 472–488. [Google Scholar] [CrossRef]
- Saoudi, M.; Messarah, M.; Boumendjel, A.; Abdelmouleh, A.; Kammoun, W.; Jamoussi, K.; Feki, A.E. Extracted tetrodotoxin from puffer fish Lagocephalus lagocephalus induced hepatotoxicity and nephrotoxicity to wistar rats. Afr. J. Biotechnol. 2011, 10, 8140–8145. [Google Scholar]
- Oliveira, F.; Monteiro, P.; Afonso, C.; Veiga, P.; Bentes, L.; Calado, R.; Gonçalves, J.M.S. First record of Calcinus tubularis on the southern coast of Portugal (Crustacea: Decapoda: Anomura: Diogenidae). Mar. Biodivers. Rec. 2011, 4, e21. [Google Scholar] [CrossRef]
- Morais, P.; Teodósio, M.A. The transatlantic introduction of weakfish Cynoscion regalis (Bloch & Schneider, 1801) (Sciaenidae, Pisces) into Europe. BioInvasions Rec. 2016, 5, 259–265. [Google Scholar]
- Morais, P.; Cerveira, I.; Teodósio, M.A. An Update on the Invasion of Weakfish Cynoscion regalis (Bloch & Schneider, 1801) (Actinopterygii: Sciaenidae) into Europe. Diversity 2017, 9, 47. [Google Scholar]
- Morais, P.; Gaspar, M.; Garel, E.; Baptista, V.; Cruz, J.; Cerveira, I.; Leitão, F.; Teodósio, M.A. The Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its non-native distribution into the Ria Formosa lagoon and the Guadiana estuary (SW-Iberian Peninsula, Europe). BioInvasions Rec. 2019, 8. in press. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).