Communities and Attachment Networks Associated with Primary, Secondary and Alternative Foundation Species; A Case Study of Stressed and Disturbed Stands of Southern Bull Kelp
Abstract
:1. Introduction
1.1. Primary, Secondary and Alternative Foundation Species
1.2. Disturbances and Stress to Southern Bull Kelps
2. Methods
2.1. Study Site, Model Organisms, Experimental Conditions and Data Clustering
2.2. Analyses of Community Structures From Abundance Data
2.3. Analyses of Community Structures From Attachment Data
3. Results
3.1. Abundances
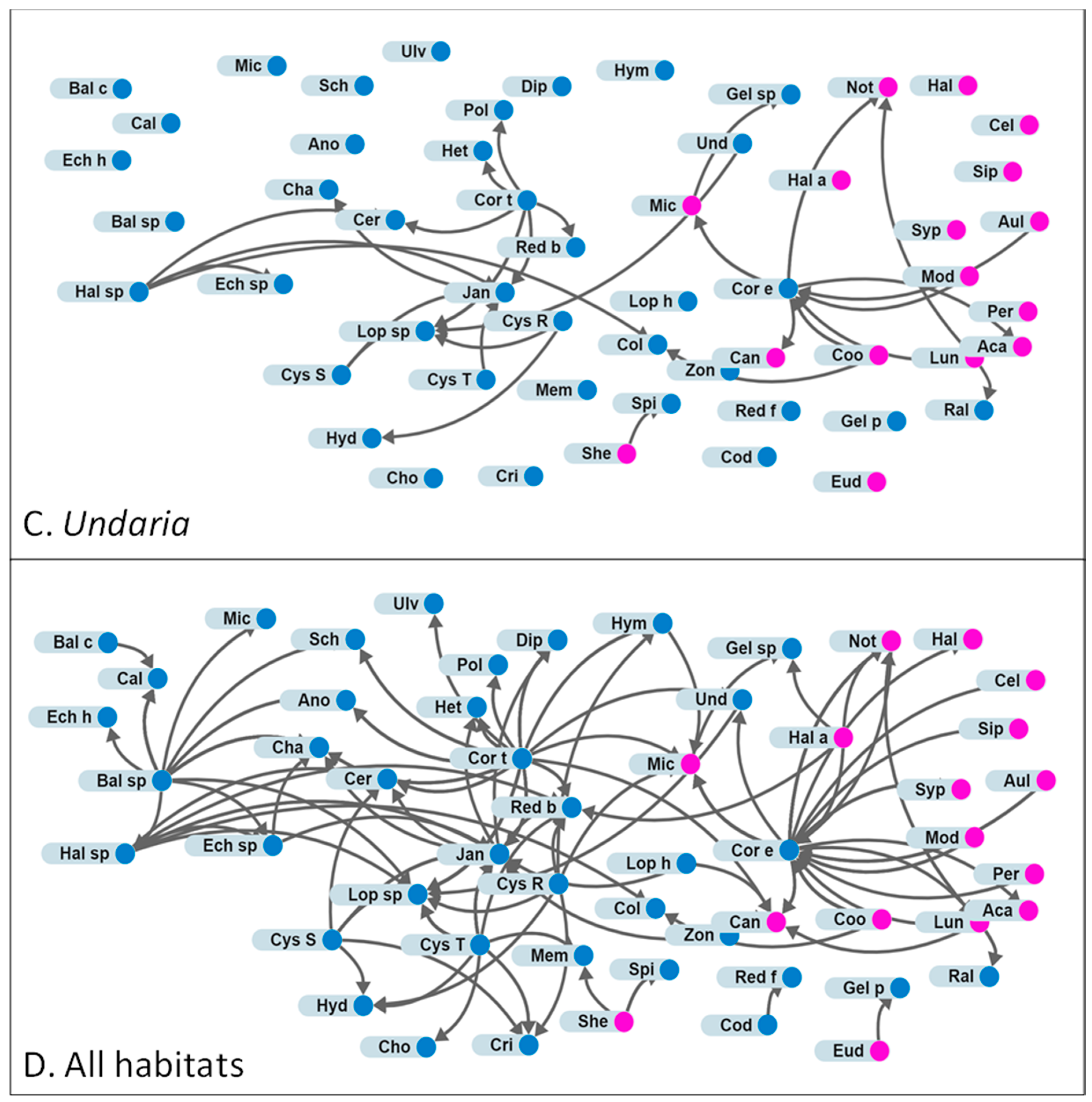
3.2. Attachments
4. Discussion
4.1. Introduction
4.2. Comparing Habitats Dominated by Durvillaea spp., Undaria pinnatifida or Cystophora spp.
4.3. The Future of Durvillaea Beds in New Zealand
4.4. Research Gaps and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dayton, P.K. Towards an understanding of community resilience and the potential effects of enrichment to the benthos of McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antartica; Parker, B.C., Ed.; Allen Press: Lawrence, KS, USA, 1972; pp. 81–96. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges for the quest for keystones. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Huston, M.A. Biological Diversity: The Coexistence of Species on Changing Landscapes; Cambridge University Press: Cambridge, UK, 1994; p. 681. [Google Scholar]
- Darwin, C. The Origin of Species; The Modern Library: New York, NY, USA, 1859; p. 686. [Google Scholar]
- Möbius, K. Die Auster und die Austernwirtschaft; Wiegundt, Hempel and Parey: Berlin, Germany, 1877; p. 126. [Google Scholar]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Yakovis, E.L.; Artemieva, A.V.; Shunatova, N.N.; Varfolomeeva, M.A. Multiple foundation species shape benthic habitat islands. Oecologia 2008, 155, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Wootton, J.T. Estimates and tests of per capita interaction strength: Diet, abundance, and impact of intertidally foraging birds. Ecol. Monogr. 1997, 67, 45–64. [Google Scholar] [CrossRef]
- Paine, R.T. Food-web analysis through field measurement of per capita interaction strength. Nature 1992, 355, 73. [Google Scholar] [CrossRef]
- Dayton, P.K. Experimental Evaluation of Ecological Dominance in a Rocky Intertidal Algal Community. Ecol. Monogr. 1975, 45, 137–159. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Gribben, P.E.; Lear, G.; He, Q.; Schiel, D.R.; Silliman, B.R.; South, P.M. Secondary foundation species enhance biodiversity. Nat. Ecol. Evol. 2018, 2, 634. [Google Scholar] [CrossRef]
- Yakovis, E.; Artemieva, A. Cockles, barnacles and ascidians compose a subtidal facilitation cascade with multiple hierarchical levels of foundation species. Sci. Rep. 2017, 7, 237. [Google Scholar] [CrossRef]
- Angelini, C.; Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BioScience 2011, 61, 782–789. [Google Scholar] [CrossRef]
- Dijkstra, J.A.; Boudreau, J.; Dionne, M. Species-specific mediation of temperature and community interactions by multiple foundation species. Oikos 2012, 121, 646–654. [Google Scholar] [CrossRef]
- Ellison, A.M.; Barker-Plotkin, A.A.; Foster, D.R.; Orwig, D.A. Experimentally testing the role of foundation species in forests The Harvard Forest Hemlock Removal Experiment. Methods Ecol. Evol. 2010, 1, 168–179. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Morris, R.J. Ecological networks across environmental gradients. Annu. Rev. Ecol. Evol. Syst. 2017, 48. [Google Scholar] [CrossRef]
- Blüthgen, N. Why network analysis is often disconnected from community ecology: A critique and an ecologist’s guide. Basic Appl. Ecol. 2010, 11, 185–195. [Google Scholar] [CrossRef]
- Dunne, J.A.; Williams, R.J.; Martinez, N.D. Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. USA 2002, 99, 12917–12922. [Google Scholar] [CrossRef] [Green Version]
- Bascompte, J.; Jordano, P. Mutualistic Networks; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Ollerton, J.; McCollin, D.; Fautin, D.G.; Allen, G.R. Finding NEMO: Nestedness engendered by mutualistic organization in anemonefish and their hosts. Proc. R. Soc. B Biol. Sci. 2006, 274, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Floeter, S.R.; Vázquez, D.P.; Grutter, A.S. The macroecology of marine cleaning mutualisms. J. Anim. Ecol. 2007, 76, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, F.; Boyer, M.; Ollerton, J. Assemblage and interaction structure of the anemonefish-anemone mutualism across the Manado region of Sulawesi, Indonesia. Environ. Biol. Fishes 2010, 87, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Quimbayo, J.P.; Cantor, M.; Dias, M.S.; Grutter, A.S.; Gingins, S.; Becker, J.H.; Floeter, S.R. The global structure of marine cleaning mutualistic networks. Glob. Ecol. Biogeogr. 2018, 27, 1238–1250. [Google Scholar] [CrossRef]
- Burns, K. Network properties of an epiphyte metacommunity. J. Ecol. 2007, 95, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Burns, K.; Zotz, G. A hierarchical framework for investigating epiphyte assemblages: Networks, meta-communities, and scale. Ecology 2010, 91, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Blick, R.; Burns, K.; Moles, A. Predicting network topology of mistletoe–host interactions: Do mistletoes really mimic their hosts? Oikos 2012, 121, 761–771. [Google Scholar] [CrossRef]
- Blick, R.; Burns, K.; Moles, A. Dominant network interactions are not correlated with resource availability: A case study using mistletoe host interactions. Oikos 2013, 122, 889–895. [Google Scholar] [CrossRef]
- Sáyago, R.; Lopezaraiza-Mikel, M.; Quesada, M.; Álvarez-Añorve, M.Y.; Cascante-Marín, A.; Bastida, J.M. Evaluating factors that predict the structure of a commensalistic epiphyte–phorophyte network. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Geekiyanage, N.; Xu, J.; Khin, M.M.; Nurdiana, D.R.; Paudel, E.; Harrison, R.D. Structure of the epiphyte community in a tropical montane forest in SW China. PloS ONE 2015, 10, e0122210. [Google Scholar] [CrossRef]
- Taylor, A.; Saldaña, A.; Zotz, G.; Kirby, C.; Díaz, I.; Burns, K. Composition patterns and network structure of epiphyte–host interactions in Chilean and New Zealand temperate forests. N. Z. J. Bot. 2016, 54, 204–222. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Evans, D.M.; Garbin, M.L.; Ruiz-Miranda, C.R. Structure and robustness of an epiphyte–phorophyte commensalistic network in a neotropical inselberg. Austral Ecol. 2018, 43, 903–914. [Google Scholar] [CrossRef]
- Zotarelli, H.G.; Molina, J.M.; Ribeiro, J.E.; Sofia, S.H. A commensal network of epiphytic orchids and host trees in an Atlantic Forest remnant: A case study revealing the important role of large trees in the network structure. Austral Ecol. 2019, 44, 114–125. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Buza-Jacobucci, G.; Pereira-Leite, F.P. The role of epiphytic algae and different species of Sargassum in the distribution and feeding of herbivorous amphipods. Lat. Am. J. Aquat. Res. 2014, 42, 353–363. [Google Scholar] [CrossRef]
- Cruz-Angon, A.; Baena, M.L.; Greenberg, R. The contribution of epiphytes to the abundance and species richness of canopy insects in a Mexican coffee plantation. J. Trop. Ecol. 2009, 45, 453–463. [Google Scholar] [CrossRef]
- Edgar, G.J.; Robertson, A.I. The influence of seagrass structure on the distribution and abundance of mobile epifauna: Pattern and processes in a Western Australian Amphibolis bed. J. Exp. Mar. Biol. Ecol. 1992, 160, 13–31. [Google Scholar] [CrossRef]
- Pettersson, R.B.; Ball, J.P.; Renhorn, K.-E.; Esseen, P.-A.; Sjöberg, K. Invertebrate communities in boreal forest canopies as influenced by forestry and lichens with implications for passerine birds. Biol. Conserv. 1995, 74, 57–63. [Google Scholar] [CrossRef]
- Viejo, R.M.; Åberg, P. Temporal and spatial variation in the density of mobile epifauna and grazing damage on the seaweed Ascophyllum nodosum. Mar. Biol. 2003, 142, 1229–1241. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Silliman, B.R. Secondary foundation species as drivers of trophic and functional diversity: Evidence from a tree–epiphyte system. Ecology 2014, 95, 185–196. [Google Scholar] [CrossRef]
- Angelini, C.; Briggs, K.L. Spillover of secondary foundation species transforms community structure and accelerates decomposition in oak savannas. Ecosystems 2015, 18, 780–791. [Google Scholar] [CrossRef]
- Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am. Nat. 2007, 169, 195–206. [Google Scholar] [CrossRef]
- Bittick, S.J.; Clausing, R.J.; Fong, C.R.; Fong, P. Bolstered physical defences under nutrient-enriched conditions may facilitate a secondary foundational algal species in the South Pacific. J. Ecol. 2016, 104, 646–653. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Hildebrand, T.; South, P.M.; Foster, T.; Siciliano, A.; Oldach, E.; Schiel, D.R. A sixth-level habitat cascade increases biodiversity in an intertidal estuary. Ecol. Evol. 2016, 6, 8291–8303. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.S.; Silliman, B.R.; Olden, J.D.; Bruno, J.P.; Bertness, M.D. Incorporating positive interactions in aquatic restoration and conservation. Front. Ecol. Environ. 2007, 5, 153–160. [Google Scholar] [CrossRef]
- Crain, C.M.; Bertness, M.D. Ecosystem engineering across environmental gradients: Implications for conservation and management. Bioscience 2006, 56, 211–218. [Google Scholar] [CrossRef]
- Posada, J.M.; Aide, T.M.; Cavelier, J. Cattle and weedy shrubs as restoration tools of tropical montane rainforest. Restor. Ecol. 2000, 8, 370–379. [Google Scholar] [CrossRef]
- Gratton, C.; Denno, R.F. Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restor. Ecol. 2005, 13, 358–372. [Google Scholar] [CrossRef]
- Rickey, M.A.; Anderson, R.C. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. J. Appl. Ecol. 2004, 41, 888–896. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Mondardini, L.; Alestra, A.; Gerrity, S.; Tait, L.; South, P.M.; Lilley, S.A.; Schiel, D.R. Local extinction of bull kelp (Durvillaea spp.) due to a marine heatwave. Front. Mar. Sci. 2019, 6, 84–92. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; de Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef] [Green Version]
- McCook, L.; Jompa, J.; Diaz-Pulido, G. Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral reefs 2001, 19, 400–417. [Google Scholar] [CrossRef]
- Rykiel, J.R.; Edward, J. Towards a definition of ecological disturbance. Aust. J. Ecol. 1985, 10, 361–365. [Google Scholar] [CrossRef]
- Borics, G.; Várbíró, G.; Padisák, J. Disturbance and stress: Different meanings in ecological dynamics? Hydrobiologia 2013, 711, 1–7. [Google Scholar] [CrossRef]
- Grime, J.P. Plant strategies and vegetation processes. In Plant Strategies and Vegetation Processes; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Beisner, B.E.; Haydon, D.T.; Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 2003, 1, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- South, P.M.; Thomsen, M.S. The ecological role of invading Undaria pinnatifida: An experimental test of the driver–passenger models. Mar. Biol. 2016, 163, 175. [Google Scholar] [CrossRef]
- Bauer, J.T. Invasive species:“back-seat drivers” of ecosystem change? Biol. Invasions 2012, 14, 1295–1304. [Google Scholar] [CrossRef]
- Bulleri, F.; Balata, D.; Bertocci, I.; Tamburello, L.; Benedetti-Cecchi, L. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: From passenger to driver of ecological change. Ecology 2010, 91, 2205–2212. [Google Scholar] [CrossRef]
- Dayton, P.K. Ecology of kelp communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Coleman, M.A.; Wernberg, T. Forgotten underwater forests: The key role of fucoids on Australian temperate reefs. Ecol. Evol. 2017, 7, 8406–8418. [Google Scholar] [CrossRef] [Green Version]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Schiel, D.R.; Foster, M.S. The population biology of large brown seaweeds: Ecological consequences of multiphase life histories in dynamic coastal environments. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 343–372. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Toohey, B.; Kendrick, G.A.; Wernberg, T.; Phillips, J.C.; Malkin, S.; Prince, J. The effects of light and thallus scour from Ecklonia radiata canopy on an associated foliose algal assemblage: The importance of photoacclimation. Mar. Biol. 2004, 144, 1019–1027. [Google Scholar] [CrossRef]
- Taylor, D.I.; Schiel, D.R. Self-replacement and community modification by the southern bull kelp Durvillaea antarctica. Mar. Ecol. Prog. Ser. 2005, 288, 87–102. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Rise of turfs: A new battlefront for globally declining kelp forests. BioScience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Airoldi, L. The effects of sedimentation on rocky coast assemblages. Oceanogr. Mar. Biol. 2003, 41, 169–171. [Google Scholar]
- Connell, S.D.; Russell, B.D. The direct effects of increasing CO2 and temperature on non-calcifying organisms: Increasing the potential for phase shifts in kelp forests. Proc. R. Soc. B Biol. Sci. 2010, 277, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Wernberg, T.; South, P.M.; Schiel, D.R. Non-native seaweeds drive changes in marine coastal communities around the world. In Seaweed phylogeography; Springer: Berlin/Heidelberg, Germany, 2016; pp. 147–185. [Google Scholar]
- Hay, C.H. Some factors affecting the upper limit of the southern bull kelp Durvillaea antarctica (Chamisso) Hariot on two New Zealand shores. J. R. Soc. N. Z. 1979, 9, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Hurd, C. Bull kelp. In The Living Reef—The Ecology of New Zealand’s Rocky Reefs; Andrew, N., Francis, M., Eds.; Craig Potton Publishing: Nelson, New Zealand, 2003; pp. 56–63. [Google Scholar]
- Hay, C.H. Biological study of Durvillaea antarctica (Chamisso) Hariot and D. Willana Lindauer in New Zealand. Ph.D. Thesis, University of Canterbury, Canterbury, New Zealand, 1977; p. 332. [Google Scholar]
- Smith, S.; Simpson, R. Effects of the ‘Nella Dan’oil spill on the fauna of Durvillaea antarctica holdfasts. Mar. Ecol. Prog. Ser. 1995, 121, 73–89. [Google Scholar] [CrossRef]
- Schiel, D.R.; Lilley, S.A.; South, P.M. Ecological tipping points for an invasive kelp in rocky reef algal communities. Mar. Ecol. Prog. Ser. 2018, 587, 93–104. [Google Scholar] [CrossRef]
- Schiel, D.R.; Alestra, T.; Gerrity, S.; Orchard, S.; Dunmore, R.A.; Pirker, J.; Lilley, S.A.; Tait, L.; Thomsen, M.S. The Kaikōura earthquake in southern New Zealand: Loss of connectivity of marine communities and the necessity of a cross-ecosystems perspective. Aquat. Conserv. Mar. Freshw. Ecosyst 2019, in press. [Google Scholar]
- Westermeier, R.; Müller, D.G.; Gómez, I.; Rivera, P.; Wenzel, H. Population biology of Durvillaea antarctica and Lessonia nigrescens (Phaeophyta) on the rocky shores of southern Chile. Mar. Ecol. Prog. Ser. 1994, 110, 187–194. [Google Scholar] [CrossRef]
- Castilla, J.C. Earthquake-caused coastal uplift and its effects on rocky intertidal kelp communities. Science 1988, 242, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Barner, A.K.; Hacker, S.D.; Menge, B.A.; Nielsen, K.J. The complex net effect of reciprocal interactions and recruitment facilitation maintains an intertidal kelp community. J. Ecol. 2016, 104, 33–43. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; South, P.M.; Schiel, D.R. To include or not to include (the invader in community analyses)? That is the question. Biol. Invasions 2016, 18, 1515–1521. [Google Scholar] [CrossRef]
- Klein, J.C.; Verlaque, M. Experimental removal of the invasive Caulerpa racemosa triggers partial assemblage recovery. J. Mar. Biol. Assoc. UK 2011, 91, 117–125. [Google Scholar] [CrossRef]
- Staehr, P.A.; Pedersen, M.F.; Thomsen, M.S.; Wernberg, T.; Krause-Jensen, D. Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar. Ecol. Prog. Ser. 2000, 207, 79–88. [Google Scholar] [CrossRef]
- Forrest, B.M.; Taylor, M.D. Assessing invasion impact: Survey design considerations and implications for management of an invasive marine plant. Biol. Invasions 2003, 4, 375–386. [Google Scholar]
- Connor, E.F.; McCoy, E.D. The statistics and biology of the species-area relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. Getting started with PRIMER v7. In PRIMER-E: Plymouth; Plymouth Marine Laboratory: Devon, UK, 2015. [Google Scholar]
- Unistat, L. Unistat Version 5.5; Unistat: London, UK, 2002. [Google Scholar]
- Pitts, A. Polinode: A web application for the collection and analysis of network data. In Proceedings of the 2016 IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining, San Francisco, CA, USA, 18–21 August 2016; pp. 1422–1425. [Google Scholar]
- Paine, R.T. A note on trophic compelxity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Schiel, D.R. Rivets or bolts? When single species count in the function of temperate rocky reef communities. J. Exp. Mar. Biol. Ecol. 2006, 338, 233–252. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M. Integration of alien plants into a native flower–pollinator visitation web. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Donatti, C.I.; Guimarães, P.R.; Galetti, M.; Pizo, M.A.; Marquitti, F.M.; Dirzo, R. Analysis of a hyper-diverse seed dispersal network: Modularity and underlying mechanisms. Ecol. Lett. 2011, 14, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Elberling, H.; Jordano, P. Temporal dynamics in a pollination network. Ecology 2008, 89, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Mark, O. The predominantly facultative nature of epibiosis: Experimental and observational evidence. Mar. Ecol. Prog. Ser. 1999, 187, 59–66. [Google Scholar] [CrossRef]
- Thompson, J.N. Specific hypotheses on the geographic mosaic of coevolution. Am. Nat. 1999, 153, S1–S14. [Google Scholar] [CrossRef]
- Thompson, J.N.; Cunningham, B.M. Geographic structure and dynamics of coevolutionary selection. Nature 2002, 417, 735. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Testing When and How Habitat Cascades Control Biodiversity of Marine Benthic Ecosystems. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2018. [Google Scholar]
- South, P.M.; Floerl, O.; Forrest, B.M.; Thomsen, M.S. A review of three decades of research on the invasive kelp Undaria pinnatifida in Australasia: An assessment of its success, impacts and status as one of the world’s worst invaders. Mar. Environ. Res. 2017, 131, 131–243. [Google Scholar] [CrossRef]
- Kiirikki, M. Experimental evidence that Fucus vesiculosus (Phaeophyta) controls filamentous algae by means of the whiplash effect. Eur. J. Phycol. 1996, 31, 61–66. [Google Scholar] [CrossRef]
- Reeves, S.; Kriegisch, N.; Johnson, C.; Ling, S. Reduced resistance to sediment-trapping turfs with decline of native kelp and establishment of an exotic kelp. Oecologia 2018, 188, 1239–1251. [Google Scholar] [CrossRef]
- da Gama, B.A.P.; Plouguerné, E.; Pereira, R.C. Chapter Fourteen—The Antifouling Defence Mechanisms of Marine Macroalgae. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 413–440. [Google Scholar]
- Buchanan, J.; Zuccarello, G.C. Utility of molecular-assisted alpha taxonomy of the genus Cystophora (Fucales, Phaeophyceae) from New Zealand and Australia. Phycologia 2018, 57, 374–384. [Google Scholar] [CrossRef]
- Adams, N.M. Seaweeds of New Zealand; Canterbury University Press: Canterbury, New Zealand, 1994. [Google Scholar]
- South, P.M.; Lilley, S.A.; Tait, L.W.; Alestra, T.; Hickford, M.J.; Thomsen, M.S.; Schiel, D.R. Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Mar. Freshw. Res. 2016, 67, 103–112. [Google Scholar] [CrossRef]
- Epstein, G.; Smale, D.A. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Alestra, T.; Brockerhoff, D.; Lilley, S.A.; South, P.M.; Schiel, D.R. Modified kelp seasonality and invertebrate diversity where an invasive kelp co-occurs with native mussels. Mar. Biol. 2018, 165, 173. [Google Scholar] [CrossRef]
- Simpson, R.; Smith, S.; Pople, A. The effects of a spillage of diesel fuel on a rocky shore in the sub-Antarctic region (Macquarie Island). Mar. Pollut. Bull. 1995, 31, 367–371. [Google Scholar] [CrossRef]
- Fraser, C.I.; Nikula, R.; Waters, J.M. Oceanic rafting by a coastal community. Proc. R. Soc. B Biol. Sci. 2011, 278, 649–655. [Google Scholar] [CrossRef]
- Anderson, M.J.; Diebel, C.E.; Blom, W.M.; Landers, T.J. Consistency and variation in kelp holdfast assemblages: Spatial patterns of biodiversity for the major phyla at different taxonomic resolutions. J. Exp. Mar. Biol. Ecol. 2005, 320, 35–56. [Google Scholar] [CrossRef]
- Connell, S.; Foster, M.; Airoldi, L. What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Kelaher, B.; Chapman, M.; Underwood, A. Spatial patterns of diverse macrofaunal assemblages in coralline turf and their associations with environmental variables. J. Mar. Biol. Assoc. UK 2001, 81, 917–930. [Google Scholar] [CrossRef]
- Stewart, J.G. Anchor species and epiphytes in intertidal algal turf. Pac. Sci. 1982, 36, 45–59. [Google Scholar]
- Liuzzi, M.G.; Gappa, J.L. Macrofaunal assemblages associated with coralline turf: Species turnover and changes in structure at different spatial scales. Mar. Ecol. Prog. Ser. 2008, 363, 147–156. [Google Scholar] [CrossRef]
- Kelaher, B.P. Influence of physical characteristics of coralline turf on associated macrofaunal assemblages. Mar. Ecol. Prog. Ser. 2002, 232, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Flukes, E.; Johnson, C.; Wright, J. Thinning of kelp canopy modifies understory assemblages: The importance of canopy density. Mar. Ecol. Prog. Ser. 2014, 514, 57–70. [Google Scholar] [CrossRef]
- Wernberg, T.; Connell, S.D. Physical disturbance and subtidal habitat structure on open rocky coasts: Effects of wave exposure, extent and intensity. J. Sea Res. 2008, 59, 237–248. [Google Scholar] [CrossRef]
- Dayton, P.K.; Currie, V.; Gerrodette, T.; Keller, B.D.; Rosenthal, R.; Tresca, D.V. Patch dynamics and stability of some California kelp communities. Ecol. Monogr. 1984, 54, 253–289. [Google Scholar] [CrossRef]
- Erlandsson, J.; Kostylev, V.; Williams, G.A. A field technique for estimating the influence of surface complexity on movement tortuosity in the tropical limpet cellana grata gould. Ophelia 1999, 50, 215–224. [Google Scholar] [CrossRef]
- Little, C. Factors governing patterns of foraging activity in littoral marine herbivorous molluscs. J. Molluscan Stud. 1989, 55, 273–284. [Google Scholar] [CrossRef]
- Taylor, D.I.; Schiel, D.R. Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology 2010, 91, 201–211. [Google Scholar] [CrossRef]
- Steneck, R.S. A limpet-coralline alga association: Adaptations and defenses between a selective herbivore and its prey. Ecology 1982, 63, 507–522. [Google Scholar] [CrossRef]
- Airoldi, L.; Ballesteros, E.; Buonuomo, R.; Van Belzen, J.; Bouma, T.; Cebrian, E.; De Clerk, O.; Engelen, A.; Ferrario, F.; Fraschetti, S. Marine forests at risk: Solutions to halt the loss and promote the recovery of Mediterranean canopy-forming seaweeds. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014. [Google Scholar]
- Cecchi, L.B.; Cinelli, F. Canopy removal experiments in Cystoseira-dominated rockpools from the Western coast of the Mediterranean (Ligurian Sea). J. Exp. Mar. Biol. Ecol. 1992, 155, 69–83. [Google Scholar] [CrossRef]
- Bulleri, F.; Cucco, A.; Dal Bello, M.; Maggi, E.; Ravaglioli, C.; Benedetti-Cecchi, L. The role of wave-exposure and human impacts in regulating the distribution of alternative habitats on NW Mediterranean rocky reefs. Estuar. Coast. Shelf Sci. 2018, 201, 114–122. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Wernberg, T.; Tuya, F.; Thomsen, M.S.; Kendrick, G.A. Turban snails as habitat for foliose algae: Contrasting geographical patterns in species richness. Mar. Freshw. Res. 2010, 61, 1237–1242. [Google Scholar] [CrossRef]
- Thyrring, J.; Thomsen, M.S.; Wernberg, T. Large-scale facilitation of a sessile community by an invasive habitat-forming snail. Helgol. Mar. Res. 2013, 67, 789. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Altieri, A.H.; Witman, J.D. Modular mobile foundation species as reservoirs of biodiversity. Ecosphere 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Ducker, S.C.; Leblanc, J.; Johansen, H.W. An epiphytic species of Jania (Corallinaceae: Rhodophyta) endemic to southern Australia. Contrib. Herb. Aust. 1976, 17, 1–8. [Google Scholar]
- Gutiérrez, J.L.; Palomo, M.G. Increased algal fouling on mussels with barnacle epibionts: A fouling cascade. J. Sea Res. 2016, 112, 49–54. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T. Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122829. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Hargrove, W.W.; Pickering, J. Pseudoreplication: A sine qua non for regional ecology. Landsc. Ecol. 1992, 6, 251–258. [Google Scholar] [CrossRef]
- Fraser, C.I.; Winter, D.J.; Spencer, H.G.; Waters, J.M. Multigene phylogeny of the southern bull-kelp genus Durvillaea (Phaeophyceae: Fucales). Mol. Phylogenetics Evol. 2010, 57, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Hay, C.H.; Spencer, H.G.; Waters, J.M. Genetic and morphological analyses of the southern bull kelp Durvillaea antarctica (Phaeophyceae: Durvilleales) in New Zealand reveal cryptic species. J. Phycol. 2009, 45, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Schiel, D.R.; Steinbeck, J.R.; Foster, M.S. Ten years of induced ocean warming causes comprehensive changes in marine benthic communities. Ecology 2004, 85, 1833–1839. [Google Scholar] [CrossRef]
- Alestra, T.; Schiel, D.R. Non-trophic responses of algal communities to nutrient enrichment: Interactions among coralline turfs, ephemeral algae and perennial fucoids. Mar. Ecol. Prog. Ser. 2015, 538, 145–156. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Bagur, M.; Palomo, M.G. Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates. Diversity 2019, 11, 17. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Silliman, B.R. A Facilitation Cascade Enhances Local Biodiversity in Seagrass Beds. Diversity 2019, 11, 30. [Google Scholar] [CrossRef]
- Thornber, C.; Jones, E.; Thomsen, M. Epibiont-marine macrophyte assemblages. In Marine Macrophytes As Foundation Species; CRC Press: Boca Raton, FL, USA, 2016; pp. 43–65. [Google Scholar]
- Zotz, G.; Hietz, P. The physiological ecology of vascular epiphytes: Current knowledge, open questions. J. Exp. Bot. 2001, 52, 2067–2078. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, M.S.; South, P.M. Communities and Attachment Networks Associated with Primary, Secondary and Alternative Foundation Species; A Case Study of Stressed and Disturbed Stands of Southern Bull Kelp. Diversity 2019, 11, 56. https://doi.org/10.3390/d11040056
Thomsen MS, South PM. Communities and Attachment Networks Associated with Primary, Secondary and Alternative Foundation Species; A Case Study of Stressed and Disturbed Stands of Southern Bull Kelp. Diversity. 2019; 11(4):56. https://doi.org/10.3390/d11040056
Chicago/Turabian StyleThomsen, Mads S., and Paul M. South. 2019. "Communities and Attachment Networks Associated with Primary, Secondary and Alternative Foundation Species; A Case Study of Stressed and Disturbed Stands of Southern Bull Kelp" Diversity 11, no. 4: 56. https://doi.org/10.3390/d11040056




