Risks of Mixtures of Oil Sands Contaminants to a Sensitive Mayfly Sentinel, Hexagenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
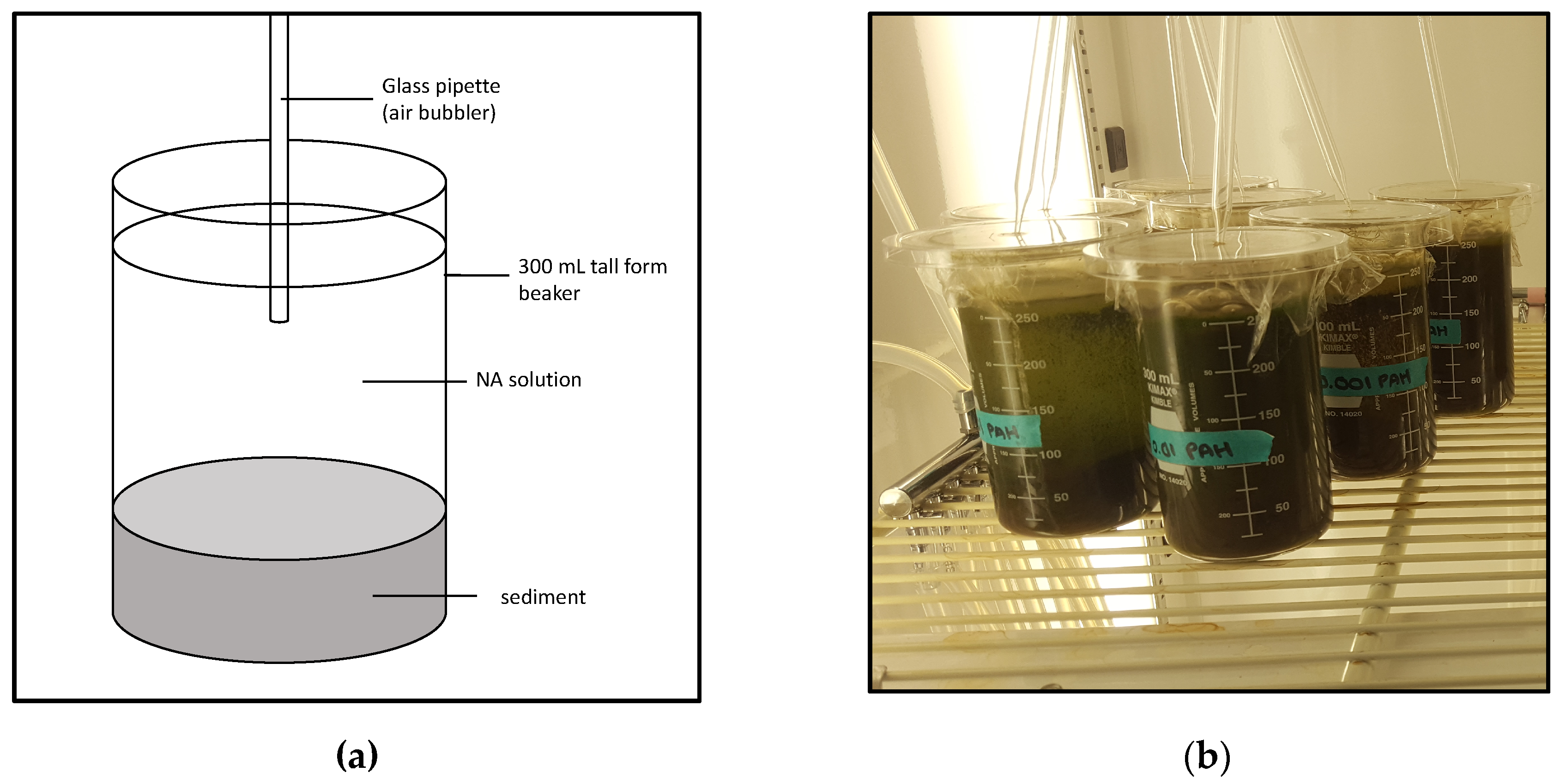
2.2. Establishment of Treatments
2.3. Chemical Analyses
2.4. Biological Response Variables
2.5. Statistical Analyses
3. Results
3.1. Lethal Responses to Treatment
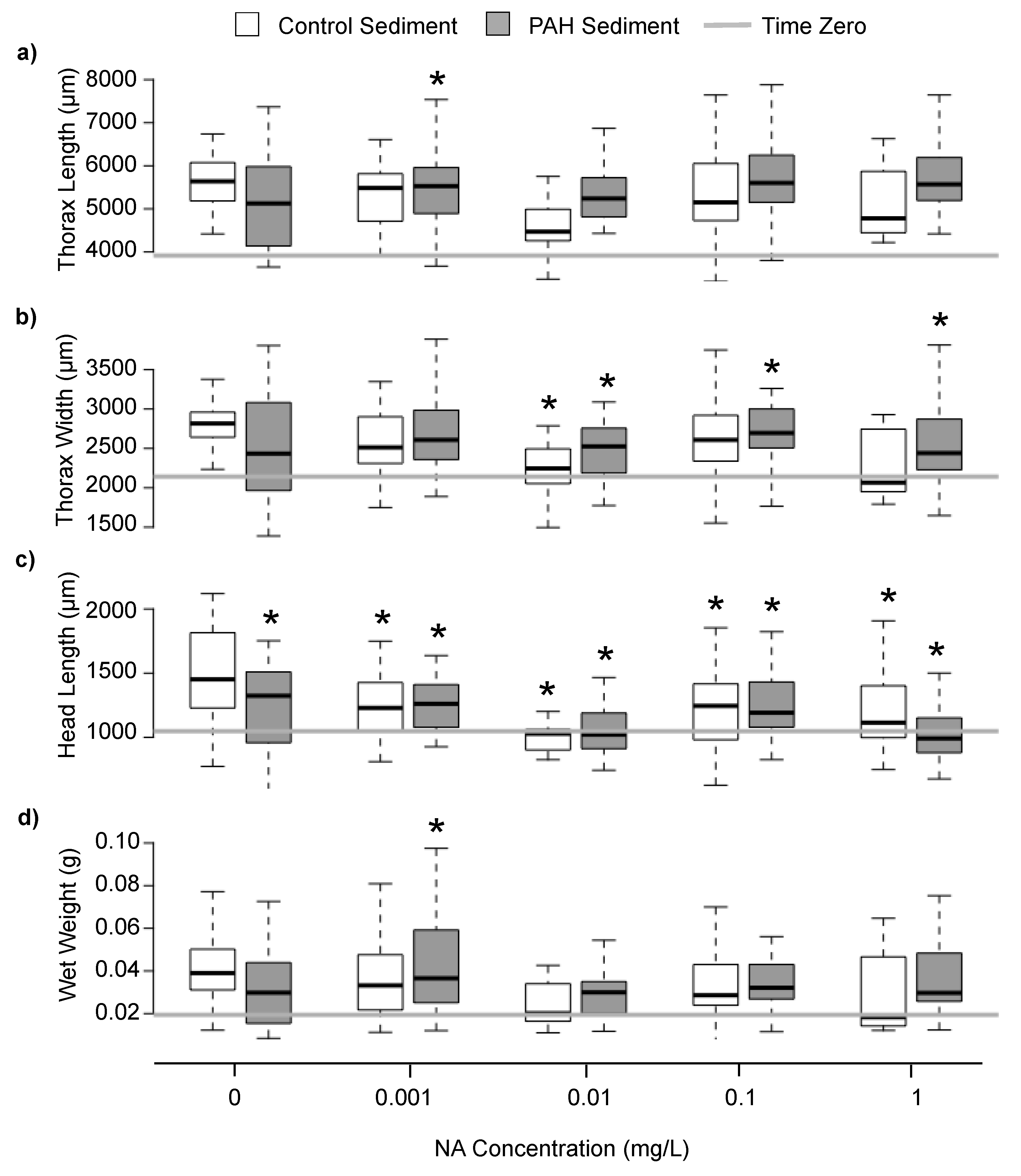
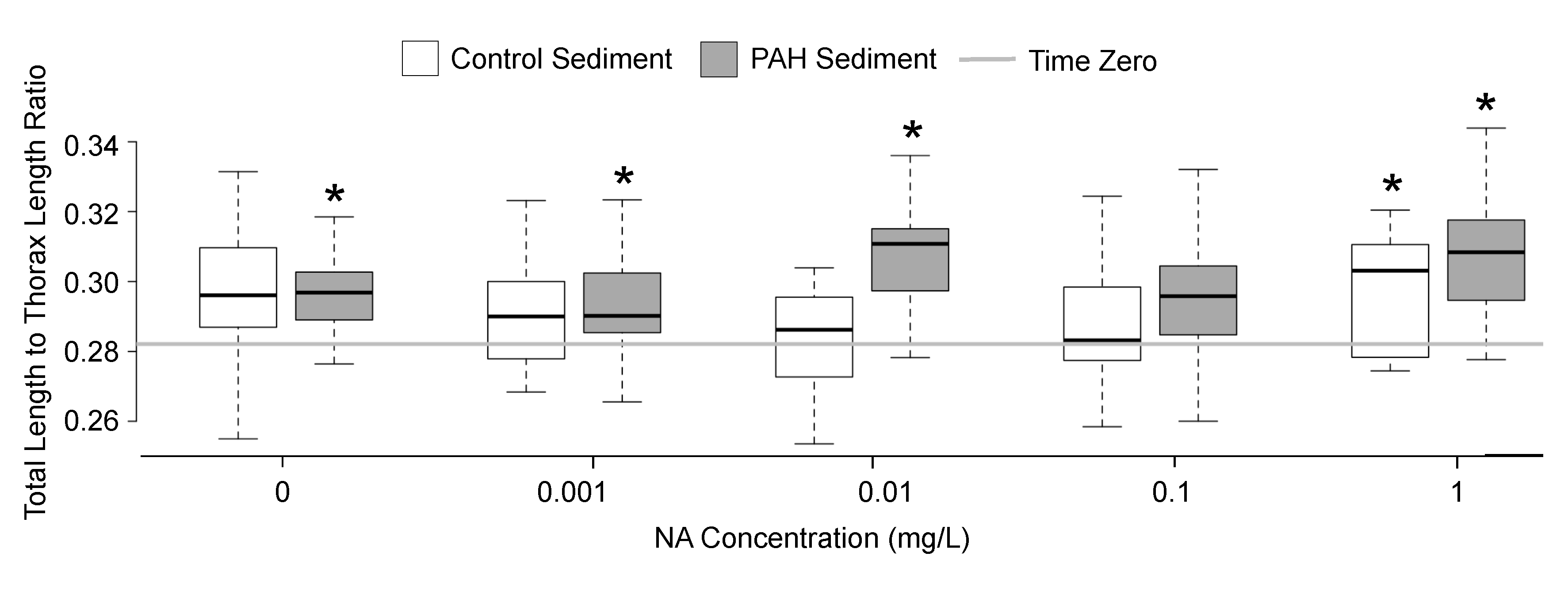
3.2. Sublethal Responses to Treatment
4. Discussion
4.1. Lethal Responses to Treatment
4.2. Sublethal Responses to Treatment
5. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CAPP (Canadian Association of Petroleum Producers). Available online: http://www.capp.ca/canadian-oil-and-natural-gas/oil-sands/what-are-oil-sands (accessed on 13 June 2019).
- Natural Resources Canada: Oil Sands Processes. Available online: https://www.nrcan.gc.ca/energy/oil-sands/58539 (accessed on 24 April 2017).
- Schramm, L.L.; Stasiuk, E.N.; MacKinnon, M. Surfactants in Athabasca Oil Sands Slurry Conditioning, Flotation, Recovery, and Tailings Processes. In Surfactants Fundamentals and Applications in the Petroleum Industry; Schramm, L.L., Ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 365–430. [Google Scholar]
- Allen, E.W. Process water treatment in Canada’s oil sands Industry: I. Target pollutants and treatment objectives. J. Environ. Eng. Sci. 2008, 7, 123–138. [Google Scholar] [CrossRef]
- Brown, L.D.; Ulrich, A.C. Oil sands naphthenic acids: A review of properties, measurement, and treatment. Chemosphere 2015, 127, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, A.; Dubé, M.; Mahaffey, A. Review of the composition and toxicity of oil sands process affected water. Environ. Rev. 2017, 25, 97–114. [Google Scholar] [CrossRef]
- Van Den Heuvel, M.R. In Response: An academic perspective on the release of oil sands process-affected water. Environ. Toxicol. Chem. 2015, 34, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, M.D.; Boerger, H. Description of two treatment methods for detoxifying oil sands tailings pond water. Water Qual. Res. J. Can. 1986, 21, 496–512. [Google Scholar] [CrossRef]
- Frank, R.A.; Roy, J.W.; Bickerton, G.; Rowland, S.J.; Headley, J.V.; Scarlett, A.G.; Hewitt, M.L. Profiling oil sands mixtures from industrial developments and natural groundwaters for source identification. Environ. Sci. Technol. 2014, 48, 2660–2670. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.R.; Alexander, A.C.; Milani, D.; Culp, J.M.; Peru, K.M. Effects of oil sands process water mixtures on the mayfly Hexagenia and field-collected aquatic macroinvertebrate communities. Ecotoxicology 2019, 28, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.; Kirk, J.L.; Muir, D.C.; Wang, X.; Evans, M.S.; Smol, J.P. Legacy of a half century of Athabasca oil sands development recorded by lake ecosystems. Proc. Natl. Acad. Sci. USA 2012, 110, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- De Hoop, L.; Schipper, A.M.; Huijbregts, M.A.J.; Hendriks, A.J.; Viaene, K.P.J.; De Laender, F. Time-varying effects of aromatic oil constituents on the survival of aquatic species: Deviations between model estimates and observations. Environ. Toxicol. Chem. 2017, 36, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.T.; Norwood, W.P.; Prepas, E.E.; Pyle, G.G. Metal-PAH mixtures in the aquatic environment: A review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat. Toxicol. 2014, 154, 253–269. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Morandi, G.; Giesy, J.P.; Wiseman, S.B. Effect of oil sands process-affected water on toxicity of retene to early life-stages of Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2016, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrel, K.R.A.; Patsinghasanee, S.; Kimura, I.; Shimizu, Y. Coupled model of bank erosion and meander evolution for cohesive riverbanks. Geosciences 2018, 8, 359. [Google Scholar] [CrossRef]
- Droppo, I.G.; di Cenzo, P.; Parrott, J.; Power, J. The Alberta oil sands eroded bitumen sediment transitional journey: Influence on sediment transport dynamics, PAH signatures and toxicological effect. Sci. Total Environ. 2019, 677, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hanes, E.C.; Ciborowski, J.J.H. Effects of density and food limitation on size variation and mortality of larval Hexagenia rigida (Ephemeroptera: Ephemeridae). Can. J. Zool. 1992, 70, 1824–1832. [Google Scholar] [CrossRef]
- Fremling, C.R.; Mauck, W.L. Methods for Using Nymphs of Burrowing Mayflies (Ephemeroptera, Hexagenia) as Toxicity Test Organisms. In Aquatic Invertebrate Bioassays; Buikema, A.L., Jr., Cairns, J., Jr., Eds.; ASTM STP 715; American Society for Testing and Materials: Philadelphia, PA, USA, 1980; pp. 81–97. [Google Scholar]
- Friesen, M.K. Manual for the Culture of Selected Freshwater Invertebrates; Lawrence, S.G., Ed.; Canadian Special Publications of Fisheries and Aquatic Sciences; Department of Fisheries and Oceans: Winnipeg, MB, Canada, 1981; Volume 54, pp. 127–142. [Google Scholar]
- Giberson, D.J.; Rosenberg, D.M. Effects of temperature, food quantity, and nymphal rearing density on life-history traits of a northern population of Hexagenia (Ephemeroptera: Ephemeridae). J. N. Am. Benthol. Soc. 1992, 11, 181–193. [Google Scholar] [CrossRef]
- Kavanaugh, R.J.; Burnison, B.K.; Frank, R.A.; Solomon, K.R.; Van Der Kraak, G. Detecting oil sands process-affected waters in the Alberta oil sands region using synchronous fluorescence spectroscopy. Chemosphere 2009, 76, 120–126. [Google Scholar] [CrossRef]
- Winer, B.J.; Brown, D.R.; Michels, K.M. Statistical Principles in Experimental Design; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Lowell, R.B.; Culp, J.M.; Wrona, F.J. Stimulation of increased short-term growth and development of mayflies by pulp mill effluent. Environ. Toxicol. Chem. 1995, 14, 1529–1541. [Google Scholar] [CrossRef]
- Alexander, A.C.; Heard, K.S.; Culp, J.M. Emergent body size of mayfly survivors. Freshw. Biol. 2008, 53, 171–180. [Google Scholar] [CrossRef]
- Mckee, D.; Atkinson, D. The influence of climate change scenarios on populations of the mayfly Cloeon dipterum. Hydrobiologia 2000, 441, 55–62. Available online: https://link-springer-com.proxy.hil.unb.ca/content/pdf/10.1023%2FA%3A1017595223819.pdf (accessed on 23 July 2019). [CrossRef]
- Scrimgeour, G.J.; Cash, K.J.; Culp, J.M. Size-dependent flight initiation by a lotic mayfly in response to a predatory fish. Freshw. Biol. 1997, 37, 91–98. [Google Scholar] [CrossRef]
- Evans, M.; Davies, M.; Janzen, K.; Muir, D.; Hazewinkel, R.; Kirk, J.; De Boer, D. PAH distributions in sediments in the oil sands monitoring area and western Lake Athabasca: Concentration, composition and diagnostic ratios. Environ. Pollut. 2016, 213, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Raine, J.C.; Turcotte, D.; Romanowski, L.; Parrott, J.L. Oil sands tailings pond sediment toxicity to early life stages of northern pike (Esox lucius). Sci. Total Environ. 2017, 664, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ajaero, C.; Peru, K.M.; Simair, M.; Friesen, V.; O’Sullivan, G.; Hughes, S.A.; McMartin, D.W.; Headley, J.V. Fate and behavior of oil sands naphthenic acids in a pilot-scale treatment wetland as characterized by negative-ion electrospray ionization Orbitrap mass spectrometry. Sci. Total Environ. 2018, 631–632, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Crosley, B.; Conly, F.M.; Quagraine, E.K. The characterization and distribution of inorganic chemicals in tributary waters of the Lower Athabasca River, Oilsands Region, Canada. J. Environ. Sci. Health 2005, 40, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.F.; Ciborowski, J.J.H.; MacKinnon, M.D. Petroleum coke and soft tailings sediment in constructed wetlands may contribute to the uptake of trace metals by algae and aquatic invertebrates. Sci. Total Environ. 2012, 414, 177–186. [Google Scholar] [CrossRef]
- Collier, K.J.; Probert, P.K.; Jeffries, M. Conservation of aquatic invertebrates: Concerns, challenges and conundrums. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 817–837. [Google Scholar] [CrossRef]
- Thorp, J.H. Chapter 4—Functional Relationships of Freshwater Invertebrates; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 65–82. [Google Scholar] [CrossRef]
- Stebbing, A.R.D. Hormesis—The stimulation of growth by low levels of inhibitors. Sci. Total Environ. 1982, 22, 213–234. [Google Scholar] [CrossRef]
- Peckarsky, B.L.; Taylor, B.W.; Mcintosh, A.R.; Mcpeek, M.A.; Lytle, D.A. Variation in Mayfly Size at Metamorphosis as a Developmental Response to Risk of. Source. Ecology 2001, 82, 740–757. Available online: https://www-jstor-org.proxy.hil.unb.ca/stable/pdf/2680193.pdf?refreqid=excelsior%3Ad5e86ade5ad420abbccfc85d077b02ab (accessed on 23 July 2019). [CrossRef]
- Giberson, D.J.; Rosenberg, D.M. Life histories of burrowing mayflies (Hexagenia limbata and H. rigida, Ephemeroptera: Ephemeridae) in a Northern Canadian reservoir. Freshw. Biol. 1994, 32, 501–551. [Google Scholar] [CrossRef]


| OSPW | Naphthenic Acids | Sodium Naphthenate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | Nominal (mg/L) | Actual (mg/L) | ±SE | Actual (µM) | ±SE | Nominal (mg/L) | Actual (mg/L) | ±SE | Actual (µM) | ±SE |
| 0.01 | 0.01 | 0.01 | <0.01 | 0.059 | <0.059 | 0.001 | <0.01 | <0.01 | <0.052 | <0.052 |
| 0.1 | 0.1 | 0.17 | <0.01 | 0.999 | <0.059 | 0.01 | 0.01 | <0.01 | 0.052 | <0.052 |
| 1 | 1 | 1.45 | 0.02 | 8.517 | 0.117 | 0.1 | 0.12 | <0.01 | 0.624 | <0.052 |
| 14 | 10 | 14.47 | 0.25 | 84.991 | 1.468 | 1 | 1.16 | 0.02 | 6.034 | 0.104 |
| PAH | This Study | Droppo Study [16] | ||
|---|---|---|---|---|
| AVG (ng/mg) | ± | SE | AVG (ng/mg) | |
| Acenaphthene | 0.174 | 0.009 | ||
| Acenaphthylene | 0.118 | 0.006 | ||
| Anthracene | 0.16 | 0.008 | ||
| Benzo(a)anthracene | 0.034 | 0.002 | ||
| Benzo(a)pyrene | 0.08 | 0.004 | 0.3322 | |
| Benzo(b)fluoranthene | 0.102 | 0.005 | ||
| Benzo(b+k)fluoranthene | 0.052 | 0.008 | ||
| Benzo(g,h,i)perylene | 0.044 | 0.002 | ||
| Benzo(k)fluoranthene | 0.063 | 0.003 | ||
| Chrysene | 0.046 | 0.002 | ||
| Dibenzo(a,h)anthracene | 0.025 | 0.001 | ||
| Fluoranthene | 0.108 | 0.005 | 0.2714 | |
| Fluorene | 0.075 | 0.004 | ||
| Indeno(1,2,3-cd) pyrene | 0.076 | 0.004 | ||
| Naphthalene | 0.094 | 0.005 | 0.4072 | |
| Phenanthrene | 0.116 | 0.006 | 0.077 | |
| Pyrene | 0.064 | 0.003 | 1.7156 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howland, J.; Alexander, A.; Milani, D.; Peru, K.; Culp, J. Risks of Mixtures of Oil Sands Contaminants to a Sensitive Mayfly Sentinel, Hexagenia. Diversity 2019, 11, 118. https://doi.org/10.3390/d11080118
Howland J, Alexander A, Milani D, Peru K, Culp J. Risks of Mixtures of Oil Sands Contaminants to a Sensitive Mayfly Sentinel, Hexagenia. Diversity. 2019; 11(8):118. https://doi.org/10.3390/d11080118
Chicago/Turabian StyleHowland, Julia, Alexa Alexander, Danielle Milani, Kerry Peru, and Joseph Culp. 2019. "Risks of Mixtures of Oil Sands Contaminants to a Sensitive Mayfly Sentinel, Hexagenia" Diversity 11, no. 8: 118. https://doi.org/10.3390/d11080118
APA StyleHowland, J., Alexander, A., Milani, D., Peru, K., & Culp, J. (2019). Risks of Mixtures of Oil Sands Contaminants to a Sensitive Mayfly Sentinel, Hexagenia. Diversity, 11(8), 118. https://doi.org/10.3390/d11080118





