Changing Trends in Cetacean Strandings in the East China Sea: Identifying Relevant Variables and Implications for Conservation and Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Stranding Data
2.2. Environmental Data
2.2.1. The Influence of Climate on Cetacean Strandings
2.2.2. The Influence of Oceanic Properties on Cetacean Strandings
2.2.3. The Influence of Human Activities on Cetacean Strandings
2.3. Data Analysis
2.3.1. Temporal and Spatial Analyses
2.3.2. Structural Equation Model
3. Results
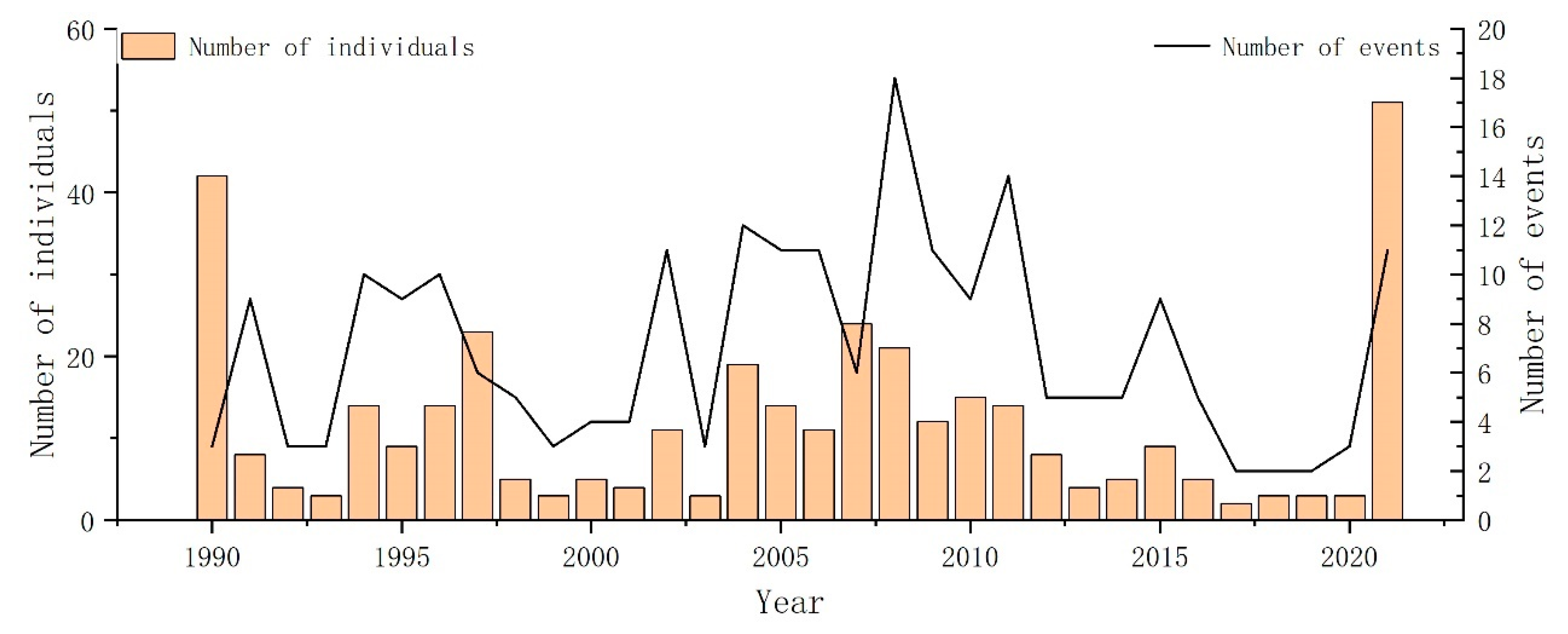
3.1. Temporal Patterns
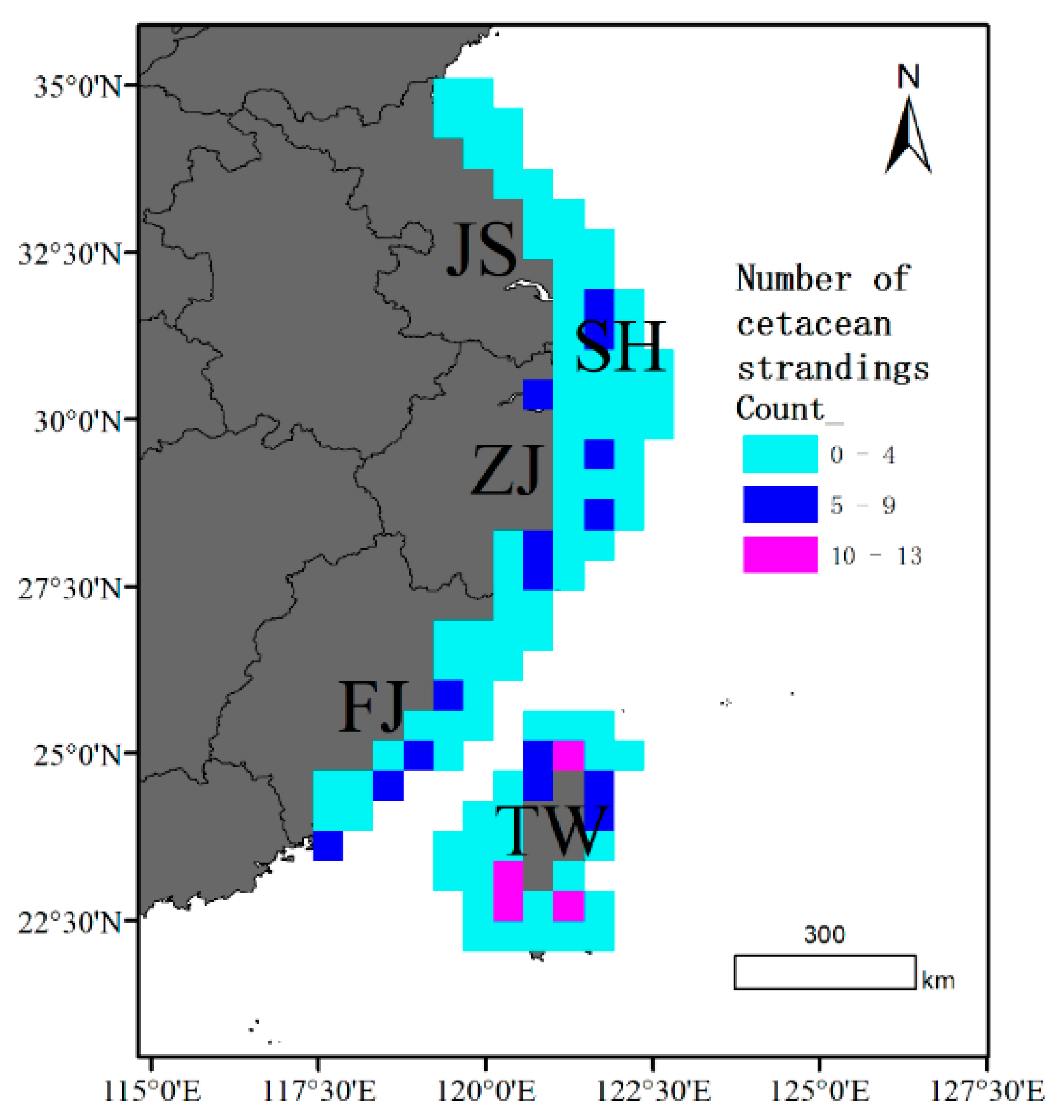
3.2. Spatial Patterns
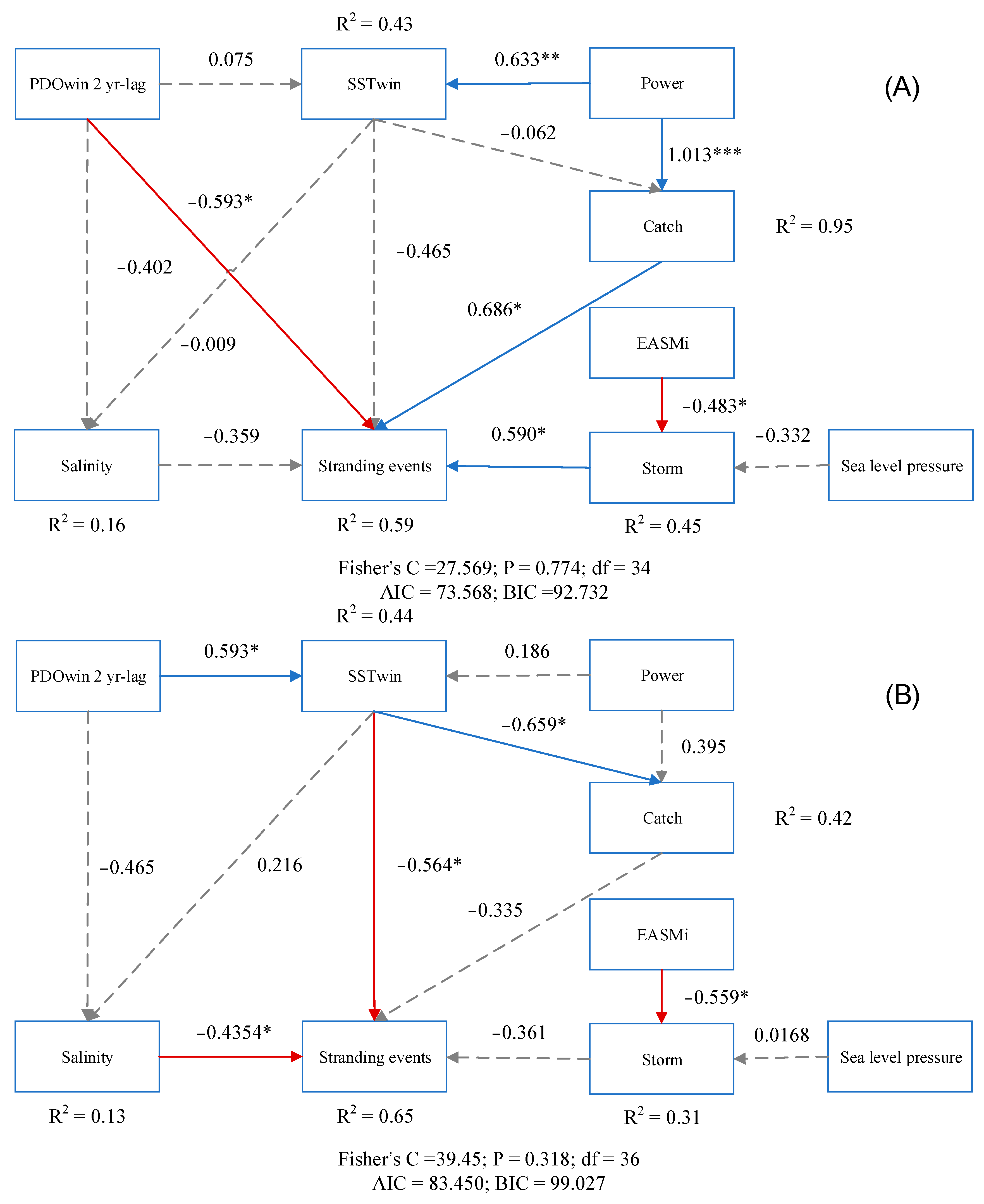
3.3. Factors Driving Patterns of Cetacean Strandings
4. Discussion
4.1. Temporal and Spatial Trends
4.2. Correlates of Strandings through Time
4.3. The Implications for Conservation and Management
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as marine ecosystem engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Wells, R.S.; Rhinehart, H.L.; Hansen, L.J.; Sweeney, J.C.; Townsend, F.I.; Stone, R.; Casper, D.R.; Scott, M.D.; Hohn, A.A.; Rowles, T.K. Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. EcoHealth 2004, 1, 246–254. [Google Scholar] [CrossRef]
- Smith, L.V.; McMinn, A.; Martin, A.; Nicol, S.; Bowie, A.R.; Lannuzel, D.; van der Merwe, P. Preliminary investigation into the stimulation of phytoplankton photophysiology and growth by whale faeces. J. Exp. Mar. Biol. Ecol. 2013, 446, 1–9. [Google Scholar] [CrossRef]
- Berge, J.; Gabrielsen, T.M.; Moline, M.; Renaud, P.E. Evolution of the Arctic Calanus complex: An Arctic marine avocado? J. Plankton Res. 2012, 34, 191–195. [Google Scholar] [CrossRef]
- Parsons, K.M.; Everett, M.; Dahlheim, M.; Park, L. Water, water everywhere: Environmental DNA can unlock population structure in elusive marine species. Roy. Soc. Open. Sci. 2018, 5, 180537. [Google Scholar] [CrossRef]
- Santos, M.C.d.O.; Bressem, M.-F.V. Cetaceans using the marine protected area of “Parque Estadual Marinho da Laje de Santos”, Southeastern Brazil. Braz. J. Oceanogr. 2017, 65, 605–613. [Google Scholar] [CrossRef]
- Bailey, H.; Thompson, P.M. Using marine mammal habitat modelling to identify priority conservation zones within a marine protected area. Mar. Ecol. Prog. Ser. 2009, 378, 279–287. [Google Scholar] [CrossRef]
- Sequeira, A.M.M.; Hays, G.C.; Sims, D.W.; Eguíluz, V.M.; Rodríguez, J.P.; Heupel, M.R.; Harcourt, R.; Calich, H.; Queiroz, N.; Costa, D.P. Overhauling ocean spatial planning to improve marine megafauna conservation. Front. Mar. Sci. 2019, 6, 639. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Tabor, G.M. Introduction: Marine vertebrates as sentinels of marine ecosystem health. EcoHealth 2004, 1, 236–238. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Halpin, P.N.; Qian, S.S.; Lawson, G.L.; Wiebe, P.H.; Thiele, D.; Read, A.J. Whale distribution in relation to prey abundance and oceanographic processes in shelf waters of the Western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2006, 317, 297–310. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Evans, P.G.; Hammond, P.S. Monitoring cetaceans in European waters. Mammal. Rev. 2004, 34, 131–156. [Google Scholar] [CrossRef]
- Hammond, P.S.; Francis, T.B.; Heinemann, D.; Long, K.J.; Moore, J.E.; Punt, A.E.; Reeves, R.R.; Sepúlveda, M.; Sigurðsson, G.M.; Siple, M.C. Estimating the abundance of marine mammal populations. Front. Mar. Sci. 2021, 8, 1316. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; ten Doeschate, M.T.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galatius, A.; Gilles, A.; Haelters, J.; Jepson, P.D.; Keijl, G.O. Spatiotemporal mortality and demographic trends in a small cetacean: Strandings to inform conservation management. Biol. Conserv. 2020, 249, 108733. [Google Scholar] [CrossRef]
- Thompson, K.F.; Millar, C.D.; Scott Baker, C.; Dalebout, M.; Steel, D.; van Helden, A.L.; Constantine, R. A novel conservation approach provides insights into the management of rare cetaceans. Biol. Conserv. 2013, 157, 331–340. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, M.; Wu, F.; Dai, Y.; Aierken, R.; Chen, M.; Wang, X. First Record of Omura’s Whale (Balaenoptera omurai) in the Beibu Gulf, China. Aquat. Mamm. 2020, 46, 301–306. [Google Scholar] [CrossRef]
- Pyenson, N.D. The high fidelity of the cetacean stranding record: Insights into measuring diversity by integrating taphonomy and macroecology. Proc. R. Soc. B 2011, 278, 3608–3616. [Google Scholar] [CrossRef]
- Quaggiotto, M.-M.; Sánchez-Zapata, J.A.; Bailey, D.M.; Payo-Payo, A.; Navarro, J.; Brownlow, A.; Deaville, R.; Lambertucci, S.A.; Selva, N.; Cortés-Avizanda, A. Past, present and future of the ecosystem services provided by cetacean carcasses. Ecosyst. Serv. 2022, 54, 101406. [Google Scholar] [CrossRef]
- Coombs, E.J.; Deaville, R.; Sabin, R.C.; Allan, L.; O’Connell, M.; Berrow, S.; Smith, B.; Brownlow, A.; Doeschate, M.T.; Penrose, R.; et al. What can cetacean stranding records tell us? A study of UK and Irish cetacean diversity over the past 100 years. Mar. Mamm. Sci. 2019, 35, 1527–1555. [Google Scholar] [CrossRef]
- Kemper, C.; Flaherty, A.; Gibbs, S.; Hill, M.; Long, M.; Byard, R. Cetacean captures, strandings and mortalities in South Australia 1881–2000, with special reference to human interactions. Aust. Mammal. 2005, 27, 37–47. [Google Scholar] [CrossRef]
- Foord, C.S.; Rowe, K.M.; Robb, K. Cetacean biodiversity, spatial and temporal trends based on stranding records (1920–2016), Victoria, Australia. PLoS ONE 2019, 14, e0223712. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.; Thresher, R.; Warneke, R.; Bradshaw, C.J.; Pook, M.; Thiele, D.; Hindell, M.A. Periodic variability in caetacean strandings: Links to large-scale climate events. Biol. Lett. 2005, 1, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D. The causes of whale strandings. N. Z. Vet. J. 1982, 30, 21–24. [Google Scholar] [CrossRef]
- Ortiz-Wolford, J.; Corona-Figueroa, M.F.; Dávila, V.; Cabrera, A.A. Cetacean stranding records along the Pacific coastline of Guatemala, 2007–2021: Implications for management, conservation and research. Mar. Policy. 2021, 134, 104827. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.; Zhang, P.; Xue, T.; Li, S. An overview of cetacean stranding around Hainan Island in the South China Sea, 1978–2016: Implications for research, conservation and management. Mar. Policy. 2019, 101, 147–153. [Google Scholar] [CrossRef]
- De Weerdt, J.; Ramos, E.A.; Pouplard, E.; Kochzius, M.; Clapham, P. Cetacean strandings along the Pacific and Caribbean coasts of Nicaragua from 2014 to 2021. Mar. Biodivers. Rec. 2021, 14, 13. [Google Scholar] [CrossRef]
- Mannino, M.A.; Talamo, S.; Tagliacozzo, A.; Fiore, I.; Nehlich, O.; Piperno, M.; Tusa, S.; Collina, C.; Di Salvo, R.; Schimmenti, V. Climate-driven environmental changes around 8200 years ago favoured increases in cetacean strandings and Mediterranean hunter-gatherers exploited them. Sci. Rep. 2015, 5, 16288. [Google Scholar] [CrossRef]
- Kebke, A.; Samarra, F.; Derous, D. Climate change and cetacean health: Impacts and future directions. Philos. T. R. Soc. B. 2022, 377, 20210249. [Google Scholar] [CrossRef]
- Russell, M.; Bloodgood, J.; Carmichael, R. Spatial, temporal and demographic patterns of cetacean strandings in the northcentral Gulf of Mexico. J. Cetacean Res. Manag. 2022, 23, 171–182. [Google Scholar] [CrossRef]
- Heinrich Vanselow, K.; Ricklefs, K.; Colijn, F. Solar driven geomagnetic anomalies and sperm whale (Physeter macrocephalus) strandings around the North Sea: An analysis of long term datasets. Open Mar. Biol. J. 2009, 3, 89–94. [Google Scholar] [CrossRef]
- Vanselow, K.H.; Jacobsen, S.; Hall, C.; Garthe, S. Solar storms may trigger sperm whale strandings: Explanation approaches for multiple strandings in the North Sea in 2016. Int. J. Astrobiol. 2018, 17, 336–344. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; Berrow, S.; Rogan, E.; O’Connor, I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 2018, 232, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef] [PubMed]
- Peltier, H.; Authier, M.; Deaville, R.; Dabin, W.; Jepson, P.D.; van Canneyt, O.; Daniel, P.; Ridoux, V. Small cetacean bycatch as estimated from stranding schemes: The common dolphin case in the northeast Atlantic. Environ. Sci. Policy. 2016, 63, 7–18. [Google Scholar] [CrossRef]
- Walker, W.A.; Coe, J.M. Survey of marine debris ingestion by odontocete cetaceans. In Proceedings of the second international conference on marine debris, Honolulu, HI, USA, 2–7 April 1989; pp. 2–7. [Google Scholar]
- Parsons, E.; Dolman, S.J.; Wright, A.J.; Rose, N.A.; Burns, W. Navy sonar and cetaceans: Just how much does the gun need to smoke before we act? Mar. Pollut. Bull. 2008, 56, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, M.; Meng, W.; Zhang, Y.; Yun, H.; Lu, Y.; Huang, Z.; Mo, X.; Hu, B.; Liu, B.; et al. Temporal-spatial change of China’s coastal ecosystems health and driving factors analysis. Sci. Total Environ. 2022, 845, 157319. [Google Scholar] [CrossRef] [PubMed]
- Committee on Taxonomy. List of Marine Mammal Species and Subspecies. Society for Marine Mammalogy. Available online: www.marinemammalscience.org (accessed on 1 September 2023).
- Liu, M.; Lin, M.; Li, S. Species diversity and spatiotemporal patterns based on cetacean stranding records in China, 1950–2018. Sci. Total Environ. 2022, 822, 153651. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, S.; Yi, Y.; Liu, s.; Wang, L.; Ding, F.; Fu, Y.; Wu, M. Define wildlife protection and promote economic animal production—Understanding of new list of key protected wild animals in china. J. Econ. Anim. 2021, 4, 25. [Google Scholar] [CrossRef]
- Li, Y. The Ministry of Agriculture and Rural Affairs of the People’s Republic of China issued a document to strengthen the protection of marine mammals. Ocean. Fish. 2021, 5, 32–33. [Google Scholar] [CrossRef]
- Aragones, L.V.; Jefferson, T.A.; Marsh, H. Marine mammal survey techniques applicable in developing countries. Asian Mar. Biol. 1997, 14, 15–39. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Q.; Miao, X.; Xu, M.; Wu, F.; Dai, Y.; Tao, C.; Mou, J.; Wang, X. An overview of cetacean strandings, bycatches and rescues along the western coast of the Taiwan Strait, China: 2010–2015. Acta Oceanolog. Sin. 2017, 36, 31–36. [Google Scholar] [CrossRef]
- Parsons, E. Strandings of small cetaceans in Hong Kong territorial waters. J. Mar. Biol. Assoc. UK 1998, 78, 1039–1042. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Geng, T. Research on the sustainable development strategy of China’s deep blue fishery economy under climate change. J. Shandong Univ. Sci. Technol. (Soc. Sci.) 2018, 6, 121–129. [Google Scholar]
- Schumann, N.; Gales, N.J.; Harcourt, R.G.; Arnould, J.P. Impacts of climate change on Australian marine mammals. Aust. J. Zool. 2013, 61, 146–159. [Google Scholar] [CrossRef]
- Santora, J.A.; Hazen, E.L.; Schroeder, I.D.; Bograd, S.J.; Sakuma, K.M.; Field, J.C. Impacts of ocean climate variability on biodiversity of pelagic forage species in an upwelling ecosystem. Mar. Ecol. Prog. Ser. 2017, 580. [Google Scholar] [CrossRef]
- Oviatt, C.; Smith, L.; McManus, M.C.; Hyde, K. Decadal Patterns of Westerly Winds, Temperatures, Ocean Gyre Circulations and Fish Abundance: A Review. Climate 2015, 3, 833–857. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; Yu, J.; Zhang, Y. Impact of climate change on China’soffshore fishing: Taking the pacific decadal oscillation as an example. Resour. Sci. 2022, 44, 386–400. [Google Scholar] [CrossRef]
- Learmonth, J.A.; MacLeod, C.D.; Santos, M.B.; Pierce, G.J.; Crick, H.; Robinson, R. Potential effects of climate change on marine mammals. Oceanogr. Mar. Biol. 2006, 44, 431. [Google Scholar] [CrossRef]
- Duignan, P.J.; Stephens, N.S.; Robb, K. Fresh water skin disease in dolphins: A case definition based on pathology and environmental factors in Australia. Sci. Rep. 2020, 10, 21979. [Google Scholar] [CrossRef]
- Bloodgood, J.C.G.; Deming, A.C.; Colegrove, K.M.; Russell, M.L.; Díaz Clark, C.; Carmichael, R.H. Causes of death and pathogen prevalence in bottlenose dolphins Tursiops truncatus stranded in Alabama, USA, between 2015 and 2020, following the Deepwater Horizon oil spill. Dis. Aquat. Org. 2023, 155, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Toms, C.N.; Stone, T.; Och, T. Skin lesion and mortality rate estimates for common bottlenose dolphin (Tursiops truncatus) in the Florida Panhandle following a historic flood. PLoS ONE 2021, 16, e0257526. [Google Scholar] [CrossRef] [PubMed]
- Gulland, F.M.; Baker, J.; Howe, M.; LaBrecque, E.; Leach, L.; Moore, S.E.; Reeves, R.R.; Thomas, P.O. A Review of Climate Change Effects on Marine Mammals in United States Waters: Past Predictions, Observed Impacts, Current Research and Conservation Imperatives. Clim. Change. Ecol. 2022, 3, 100054. [Google Scholar] [CrossRef]
- Law, R.J.; Barry, J.; Barber, J.L.; Bersuder, P.; Deaville, R.; Reid, R.J.; Brownlow, A.; Penrose, R.; Barnett, J.; Loveridge, J. Contaminants in cetaceans from UK waters: Status as assessed within the Cetacean Strandings Investigation Programme from 1990 to 2008. Mar. Pollut. Bull. 2012, 64, 1485–1494. [Google Scholar] [CrossRef]
- Bearzi, G.; Politi, E.; Agazzi, S.; Azzellino, A. Prey depletion caused by overfishing and the decline of marine megafauna in eastern Ionian Sea coastal waters (central Mediterranean). Biol. Conserv. 2006, 127, 373–382. [Google Scholar] [CrossRef]
- Leeney, R.H.; Amies, R.; Broderick, A.C.; Witt, M.J.; Loveridge, J.; Doyle, J.; Godley, B.J. Spatio-temporal analysis of cetacean strandings and bycatch in a UK fisheries hotspot. Biodivers. Conserv. 2008, 17, 2323–2338. [Google Scholar] [CrossRef]
- Trapletti, A.; Hornik, K.; LeBaron, B. Time Series Analysis and Computational Finance. 2015. Available online: http://cran.r-project.org/web/packages/tseries/index.html (accessed on 3 September 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 3 September 2023).
- Pohlert, T. Non-parametric trend tests and change-point detection. CC BY-ND 2016, 4, 1–18. [Google Scholar]
- Mustika, P.L.K.; High, K.K.; Putra, M.I.H.; Sahri, A.; Ratha, I.M.J.; Prinanda, M.O.; Agung, F.; Purnomo, F.S.; Kreb, D. When and Where Did They Strand? The Spatio-Temporal Hotspot Patterns of Cetacean Stranding Events in Indonesia. Oceans 2022, 3, 509–526. [Google Scholar] [CrossRef]
- Carpenter, T.E. Methods to investigate spatial and temporal clustering in veterinary epidemiology. Prev. Vet. Med. 2001, 48, 303–320. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling; Guilford Publications: New York, NY, USA, 2015. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods. Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Katz, M.H. Multivariable Analysis: A Practical Guide for Clinicians and PublicHealth Researchers; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Shipley, B. Confirmatory path analysis in a generalized multilevel context. Ecology 2009, 90, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, S.; Coumou, D. The role of the Pacific Decadal Oscillation and ocean-atmosphere interactions in driving US temperature predictability. npj Clim. Atmos. Sci. 2022, 5, 18. [Google Scholar] [CrossRef]
- Matsumura, S.; Horinouchi, T. Pacific Ocean decadal forcing of long-term changes in the western Pacific subtropical high. Sci. Rep. 2016, 6, 37765. [Google Scholar] [CrossRef]
- Wursig, B.; Perrin, W.F. Encyclopedia of Marine Mammals; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Truchon, M.-H.; Measures, L.; L’Hérault, V.; Brethes, J.-C.; Galbraith, P.S.; Harvey, M.; Lessard, S.; Starr, M.; Lecomte, N. Marine mammal strandings and environmental changes: A 15-year study in the St. Lawrence ecosystem. PLoS ONE 2013, 8, e59311. [Google Scholar] [CrossRef]
- Groom, C.J.; Coughran, D.K.; Smith, H.C. Records of beaked whales (family Ziphiidae) in Western Australian waters. Mar. Biodivers. Rec. 2014, 7, e50. [Google Scholar] [CrossRef]
- Mitchell, E. Northeast Pacific stranding distribution and seasonality of Cuvier’s beaked whale Ziphius cavirostris. Can. J. Zool. 1968, 46, 265–279. [Google Scholar] [CrossRef]
- Owen, K.; Kavanagh, A.S.; Warren, J.D.; Noad, M.J.; Donnelly, D.; Goldizen, A.W.; Dunlop, R.A. Potential energy gain by whales outside of the Antarctic: Prey preferences and consumption rates of migrating humpback whales (Megaptera novaeangliae). Polar Biol. 2017, 40, 277–289. [Google Scholar] [CrossRef]
- Norman, S.A.; Huggins, J.; Carpenter, T.E.; Case, J.T.; Lambourn, D.M.; Rice, J.; Calambokidis, J.; Gaydos, J.K.; Hanson, M.B.; Duffield, D.A. The application of GIS and spatiotemporal analyses to investigations of unusual marine mammal strandings and mortality events. Mar. Mamm. Sci. 2012, 28, E251–E266. [Google Scholar] [CrossRef]
- Norman, S.; Bowlby, C.; Brancato, M.; Calambokidis, J.; Duffield, D.; Gearin, P.; Gornall, T.; Gosho, M.; Hanson, B.; Hodder, J. Cetacean strandings in Oregon and Washington between 1930 and 2002. J. Cetacean Res. Manag. 2004, 6, 87–100. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.; Turvey, S.; Li, S. Fishers’ knowledge as an information source to investigate bycatch of marine mammals in the South China Sea. Anim. Conserv. 2017, 20, 182–192. [Google Scholar] [CrossRef]
- Kaiya, Z.; Leatherwood, S.; Jefferson, T.A. Records of small cetaceans in Chinese waters: A review. Asian Mar. Biol. 1995, 12, 119–139. [Google Scholar]
- Dong, J.; Wang, G.; Xiao, Z. Migration and population difference of the finless porpoise in China. Mar. Sci. 1993, 5, 42–45. [Google Scholar]
- Wang, P. A research of Odontoceti cetaceans in the Yellow Sea and Bohai Sea. Chin. J. Zool. 1979, 2, 31–34. [Google Scholar]
- Wang, Y.; Li, W.; Van Waerebeek, K. Strandings, bycatches and injuries of aquatic mammals in China, 2000–2006, as reviewed from official documents: A compelling argument for a nationwide strandings programme. Mar. Policy. 2015, 51, 242–250. [Google Scholar] [CrossRef]
- Nemiroff, L.; Wimmer, T.; Daoust, P.-Y.; McAlpine, D.F. Cetacean strandings in the Canadian Maritime provinces, 1990–2008. Can. Field. Nat. 2010, 124, 32–44. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Song, P.; Zhang, R.; Li, Y.; Zhong, Z.; Lin, L. Composition of the Taiwan Strait fish fauna. Biodivers. Sci. 2014, 22, 525. [Google Scholar] [CrossRef]
- Li, S. Studies on sea mammals and its distribution from Fujian coastal waters. J. Oceanogr. Taiwan Strait 1997, 16, 485–488. [Google Scholar]
- Li, W.-T.; Chou, L.-S.; Chiou, H.-Y.; Chen, I.-H.; Yang, W.-C. Analyzing 13 years of cetacean strandings: Multiple stressors to cetaceans in Taiwanese waters and their implications for conservation and future research. Front. Mar. Sci. 2021, 8, 606722. [Google Scholar] [CrossRef]
- Aniceto, A.S.; Tassara, L.; Rikardsen, A.; Blévin, P. Mass strandings of seven toothed and baleen whale species in Northern Norway in March 2020 call for further investigation. Polar Biol. 2021, 44, 1457–1461. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings; National Aquarium in Baltimore: Baltimore, MD, USA, 2005. [Google Scholar]
- Moore, M.J.; Mitchell, G.H.; Rowles, T.K.; Early, G. Dead cetacean? Beach, bloat, float, sink. Front. Mar. Sci. 2020, 7, 333. [Google Scholar] [CrossRef]
- Murthy, C.; Sinha, P.; Rao, Y.; Dube, S.; Rao, A.; Sinha, P.; Chittibabu, P. Storm surges: Worst coastal marine hazard. In Modelling and Monitoring of Coastal Marine Processes; Capital Publishing Company: New Delhi, India, 2008; pp. 125–140. [Google Scholar] [CrossRef]
- Lawler, I.R.; Parra, G.; Noad, M. Vulnerability of marine mammals in the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef: A Vulnerability Assessment; The Great Barrier Reef Marine Park Authority: Townsville City, Australia, 2007. [Google Scholar] [CrossRef]
- Weir, C.R.; Pierce, G.J. A review of the human activities impacting cetaceans in the eastern tropical Atlantic. Mammal. Rev. 2013, 43, 258–274. [Google Scholar] [CrossRef]
- Giralt Paradell, O.; Methion, S.; Rogan, E.; Díaz López, B. Modelling ecosystem dynamics to assess the effect of coastal fisheries on cetacean species. J. Environ. Manag. 2021, 285, 112175. [Google Scholar] [CrossRef]
- Pennino, M.G.; Rufener, M.-C.; Giménez, J.; Berlinguer, F.; Bollo, E.; Appino, S.; Zucca, D.; Chessa, G.; Rotta, A. Understanding the causes of mortality and contaminant loads of stranded cetacean species in Sardinian waters (Italy) using Bayesian Hierarchical Models. J. Sea Res. 2022, 181, 102170. [Google Scholar] [CrossRef]
- Read, A.J. The looming crisis: Interactions between marine mammals and fisheries. J. Mammal. 2008, 89, 541–548. [Google Scholar] [CrossRef]
- Reeves, R.R.; McClellan, K.; Werner, T.B. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger. Species. Res. 2013, 20, 71–97. [Google Scholar] [CrossRef]
- Hamner, R.M.; Constantine, R.; Oremus, M.; Stanley, M.; Brown, P.; Scott Baker, C. Long-range movement by Hector’s dolphins provides potential genetic enhancement for critically endangered Maui’s dolphin. Mar. Mamm. Sci. 2014, 30, 139–153. [Google Scholar] [CrossRef]
- Jaramillo-Legorreta, A.M.; Cardenas-Hinojosa, G.; Nieto-Garcia, E.; Rojas-Bracho, L.; Thomas, L.; Ver Hoef, J.M.; Moore, J.; Taylor, B.; Barlow, J.; Tregenza, N. Decline towards extinction of Mexico’s vaquita porpoise (Phocoena sinus). Roy. Soc. Open. Sci. 2019, 6, 190598. [Google Scholar] [CrossRef]
- Ambie, S.; Peter, C.; Minton, G.; Ngeian, J.; Zulkifli Poh, A.N.; Mujahid, A.; Tuen, A.A. Utilizing interview-based data to measure interactions of artisanal fishing communities and cetacean populations in Kuching Bay, Sarawak, East Malaysia. Ocean. Coast. Manag. 2023, 239, 106592. [Google Scholar] [CrossRef]
- Benaka, L.; Bullock, D.; Davis, J.; Seney, E.; Winarsoo, H. US National Bycatch Report (Update 1); US Department of Commerce: Washington, DC, USA, 2013. [Google Scholar]
- Gilman, E.; Brothers, N.; McPherson, G.; Dalzell, P. A review of cetacean interactions with longline gear. J. Cetacean Res. Manag. 2007, 8, 215. [Google Scholar] [CrossRef]
- Tulloch, V.; Pirotta, V.; Grech, A.; Crocetti, S.; Double, M.; How, J.; Kemper, C.; Meager, J.; Peddemors, V.; Waples, K. Long-term trends and a risk analysis of cetacean entanglements and bycatch in fisheries gear in Australian waters. Biodivers. Conserv. 2020, 29, 251–282. [Google Scholar] [CrossRef]
- de Quirós, Y.B.; Hartwick, M.; Rotstein, D.S.; Garner, M.M.; Bogomolni, A.; Greer, W.; Niemeyer, M.E.; Early, G.; Wenzel, F.; Moore, M. Discrimination between bycatch and other causes of cetacean and pinniped stranding. Dis. Aquat. Org. 2018, 127, 83–95. [Google Scholar] [CrossRef]
- Díaz López, B.; Methion, S.; Giralt Paradell, O. Living on the edge: Overlap between a marine predator’s habitat use and fisheries in the Northeast Atlantic waters (NW Spain). Prog. Oceanogr. 2019, 175, 115–123. [Google Scholar] [CrossRef]
- Evans, P.G.H. Chapter 5—Conservation threats. In European Whales, Dolphins, and Porpoises; Evans, P.G.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 159–202. [Google Scholar]
- Board, O.S.; Council, N.R. Marine Mammal Populations and Ocean Noise: Determining When Noise Causes Biologically Significant Effects; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Zhang, W.; Dong, X.; Xue, F. Intraseasonal variations of the East Asian Summer Monsoon in El Niño developing years and La Niña years under different phases of the Pacific Decadal Oscillation. Chin. J. Atmos. Sci. 2020, 44, 390–406. [Google Scholar]
- Saavedra, C.; Pierce, G.J.; Gago, J.; Jusufovski, D.; Cabrero, Á.; Cerviño, S.; López, A.; Martínez-Cedeira, J.A.; Santos, M.B. Factors driving patterns and trends in strandings of small cetaceans. Mar. Biol. 2017, 164, 165. [Google Scholar] [CrossRef]
- Warlick, A.J.; Huggins, J.L.; Lambourn, D.M.; Duffield, D.A.; D’Alessandro, D.N.; Rice, J.M.; Calambokidis, J.; Hanson, M.B.; Gaydos, J.K.; Jeffries, S.J.; et al. Cetacean strandings in the US Pacific northwest 2000–2019 reveal potential linkages to oceanographic variability. Front. Mar. Sci. 2022, 9, 758812. [Google Scholar] [CrossRef]
- MacLeod, C.D.; Bannon, S.M.; Pierce, G.J.; Schweder, C.; Learmonth, J.A.; Herman, J.S.; Reid, R.J. Climate change and the cetacean community of north-west Scotland. Biol. Conserv. 2005, 124, 477–483. [Google Scholar] [CrossRef]
- MacLeod, C.D. Global climate change, range changes and potential implications for the conservation of marine cetaceans: A review and synthesis. Endanger. Species Res. 2009, 7, 125–136. [Google Scholar] [CrossRef]
- Wild, S.; Krützen, M.; Rankin, R.W.; Hoppitt, W.J.; Gerber, L.; Allen, S.J. Long-term decline in survival and reproduction of dolphins following a marine heatwave. Curr. Biol. 2019, 29, R239–R240. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Hessing, S.; Mairo, A.; Ten Doeschate, M.T.; Treep, J.; van den Broek, J.; Keijl, G.O.; Siebert, U.; Heesterbeek, H.; Gröne, A. Nutritional status and prey energy density govern reproductive success in a small cetacean. Sci. Rep. 2021, 11, 19201. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food. Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Velasco, J.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Sánchez-Fernández, D.; Arribas, P.; Carbonell, J.A.; Millán, A.; Pallarés, S. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos. T. R. Soc. B. 2019, 374, 20180011. [Google Scholar] [CrossRef] [PubMed]
- Cates, K.; DeMaster, D.; Brownell Jr, R.; Silber, G.; Gende, S.; Leaper, R. Strategic Plan to Mitigate the Impacts of Ship Strikes on Cetacean Populations: 2017–2020, IWC Strategic Plan to Mitigate Ship Strikes. Available online: https://iwc.int/ship-strikes (accessed on 6 September 2023).
- Laist, D.W.; Knowlton, A.R.; Mead, J.G.; Collet, A.S.; Podesta, M. Collisions between ships and whales. Mar. Mamm. Sci. 2001, 17, 35–75. [Google Scholar] [CrossRef]
- Peltier, H.; Beaufils, A.; Cesarini, C.; Dabin, W.; Dars, C.; Demaret, F.; Dhermain, F.; Doremus, G.; Labach, H.; Van Canneyt, O. Monitoring of marine mammal strandings along French coasts reveals the importance of ship strikes on large cetaceans: A challenge for the European Marine Strategy Framework Directive. Front. Mar. Sci. 2019, 6, 486. [Google Scholar] [CrossRef]
- Douglas, A.B.; Calambokidis, J.; Raverty, S.; Jeffries, S.J.; Lambourn, D.M.; Norman, S.A. Incidence of ship strikes of large whales in Washington State. J. Mar. Biol. Assoc. UK 2008, 88, 1121–1132. [Google Scholar] [CrossRef]
- Martínez-Cedeira, J.; Morales, X.; Garcia, J.; Parada, A.; Covelo, P.; López, A. How many strand? Offshore marking and coastal recapture of cetacean carcasses. In Proceedings of the Abstract Book—25th Conference of the European Cetacean Society, Cadiz, Spain, 21–23 March 2011. [Google Scholar]
- Peltier, H.; Dabin, W.; Daniel, P.; Van Canneyt, O.; Dorémus, G.; Huon, M.; Ridoux, V. The significance of stranding data as indicators of cetacean populations at sea: Modelling the drift of cetacean carcasses. Ecol. Indic. 2012, 18, 278–290. [Google Scholar] [CrossRef]
- Betty, E.L.; Bollard, B.; Murphy, S.; Ogle, M.; Hendriks, H.; Orams, M.B.; Stockin, K.A. Using emerging hot spot analysis of stranding records to inform conservation management of a data-poor cetacean species. Biodivers. Conserv. 2020, 29, 643–665. [Google Scholar] [CrossRef]
- Pradip Na Thalang, P.; Thongratsakul, S.; Poolkhet, C. Spatial, Temporal, and Geographical Factors Associated with Stranded Marine Endangered Species in Thailand during 2006–2015. Biology 2023, 12, 448. [Google Scholar] [CrossRef]
- Dudhat, S.; Pande, A.; Nair, A.; Mondal, I.; Srinivasan, M.; Sivakumar, K. Spatio-temporal analysis identifies marine mammal stranding hotspots along the Indian coastline. Sci. Rep. 2022, 12, 4128. [Google Scholar] [CrossRef]
- Peltier, H.; Ridoux, V. Marine megavertebrates adrift: A framework for the interpretation of stranding data in perspective of the European Marine Strategy Framework Directive and other regional agreements. Environ. Sci. Policy. 2015, 54, 240–247. [Google Scholar] [CrossRef]
- Ganley, L.C.; Byrnes, J.; Pendleton, D.E.; Mayo, C.A.; Friedland, K.D.; Redfern, J.V.; Turner, J.T.; Brault, S. Effects of changing temperature phenology on the abundance of a critically endangered baleen whale. Glob. Ecol. Conserv. 2022, 38, e02193. [Google Scholar] [CrossRef]
- Prado, J.H.F.; Mattos, P.H.; Silva, K.G.; Secchi, E.R. Long-Term Seasonal and Interannual Patterns of Marine Mammal Strandings in Subtropical Western South Atlantic. PLoS ONE 2016, 11, e0146339. [Google Scholar] [CrossRef] [PubMed]
- Spitz, J.; Richard, E.; Meynier, L.; Pusineri, C.; Ridoux, V. Dietary plasticity of the oceanic striped dolphin, Stenella coeruleoalba, in the neritic waters of the Bay of Biscay. J. Sea Res. 2006, 55, 309–320. [Google Scholar] [CrossRef]



| Variable | Data | Sources * |
|---|---|---|
| Pacific Decadal Oscillation | A pattern of climate variability in the Pacific Ocean characterized by long-term changes in sea surface temperatures and atmospheric conditions | Climate Prediction Center, NOAA |
| The East Asian summer monsoon index | A measure of the strength and activity of the summer monsoon in East Asia | National Tibetan Plateau Data Center |
| Sea Level Pressure | The atmospheric pressure at sea level | The Fleet Numerical Meteorology and Oceanography Center (FNMOC), NOAA |
| Storm surge | Count of annual storm surge occurrences | Bulletin of China marine disaster |
| Sea surface temperature | The measurement of ocean surface temperature | Met Office: HadISST |
| Sea surface Salinity | The measurement of salt content in the uppermost layer of the ocean’s surface | Naval Oceanographic Office, NOAA NCEI |
| Power of fishing vessel | Annual total power of fishing vessels | China Fisheries Yearbook from 1990–2021 |
| Yearly fishing catch | Total yearly catch data for 40 fish species | China Fisheries Yearbook from 1990–2021 |
| Common Name | Scientific Name | Family | Number of Events |
|---|---|---|---|
| Short-finned pilot whale | Globicephala macrorhynchus Gray, 1846 | Delphinidae | 27 |
| Risso’s dolphin | Grampus griseus G. Cuvier, 1812 | Delphinidae | 14 |
| Pygmy killer whale | Feresa attenuata Gray, 1874 | Delphinidae | 9 |
| Indo-Pacific humpback dolphin | Sousa chinensis Osbeck, 1765 | Delphinidae | 9 |
| Pantropical spotted dolphin | Stenella attenuata Gray, 1846 | Delphinidae | 9 |
| Melon-headed whale | Peponocephala electra Gray, 1846 | Delphinidae | 7 |
| Fraser’s dolphin | Lagenodelphis hosei Fraser, 1956 | Delphinidae | 6 |
| Rough-toothed dolphin | Steno bredanensis G. Cuvier in Lesson, 1828 | Delphinidae | 5 |
| Common bottlenose dolphin | Tursiops truncatus Montagu, 1821 | Delphinidae | 5 |
| False killer whale | Pseudorca crassidens Owen, 1846 | Delphinidae | 3 |
| Spinner dolphin | Stenella longirostris Gray, 1828 | Delphinidae | 2 |
| Common minke whale | Balaenoptera acutorostrata Lacépède, 1804 | Balaenopteridae | 13 |
| Omura‘s whale | Balaenoptera omurai Wada, Oishi & Yamada, 2003 | Balaenopteridae | 12 |
| Bryde’s whale | Balaenoptera edeni Anderson, 1878 | Balaenopteridae | 11 |
| Fin whale | Balaenoptera physalus Linnaeus, 1758 | Balaenopteridae | 4 |
| Sei whale | Balaenoptera borealis Lesson, 1828 | Balaenopteridae | 3 |
| Humpback whale | Megaptera novaeangliae Borowski, 1781 | Balaenopteridae | 3 |
| Cuvier’s beaked whale | Ziphius cavirostris Cuvier, 1823 | Ziphiidae | 10 |
| Blainville’s beaked whale | Mesoplodon densirostris de Blainville, 1817 | Ziphiidae | 9 |
| Longman’s beaked whale | Mesoplodon pacificus Longman, 1926 | Ziphiidae | 2 |
| Ginkgo-toothed beaked whale | Mesoplodon ginkgodens Baker & van Helden, 1999 | Ziphiidae | 1 |
| Narrow-ridged finless porpoise | Neophocaena asiaeorientalis Pilleri and Gihr, 1972 | Phocoenidae | 40 |
| Indo-Pacific finless porpoise | Neophocaena phocaenoides Cuvier, 1829 | Phocoenidae | 2 |
| Dwarf sperm whale | Kogia sima Owen, 1866 | Kogiidae | 13 |
| Pygmy sperm whale | Kogia breviceps de Blainville, 1838 | Kogiidae | 9 |
| Sperm whale | Physeter macrocephalus Linnaeus, 1758 | Physeteridae | 9 |
| North Pacific right whale | Eschrichtius robustus Lilljeborg, 1861 | Eschrichtiidae | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Li, S.; Jin, Y.; Liu, Z. Changing Trends in Cetacean Strandings in the East China Sea: Identifying Relevant Variables and Implications for Conservation and Management. Diversity 2023, 15, 1082. https://doi.org/10.3390/d15101082
Yang S, Li S, Jin Y, Liu Z. Changing Trends in Cetacean Strandings in the East China Sea: Identifying Relevant Variables and Implications for Conservation and Management. Diversity. 2023; 15(10):1082. https://doi.org/10.3390/d15101082
Chicago/Turabian StyleYang, Shaobo, Shengfa Li, Yan Jin, and Zunlei Liu. 2023. "Changing Trends in Cetacean Strandings in the East China Sea: Identifying Relevant Variables and Implications for Conservation and Management" Diversity 15, no. 10: 1082. https://doi.org/10.3390/d15101082






