Abstract
Aedes species are vectors of the most significant arboviruses in Brazil with the greatest health and economic impact in the country. However, little is known about the factors that influence the distribution of Aedes, as well as other container-breeding mosquitoes, especially on the border between urban and forest environments. Here, we tested the effect of three phytophysiognomies within the city–forest ecotone in the Brazilian semi-arid region on the spatial distribution of vector mosquitoes. We also investigated the effect of rainfall on the spatial distribution and abundance of mosquitoes and described the interspecific interactions between invasive and native mosquito species. Immatures were sampled during the rainy seasons of 2019–2020 (October 2019 to February 2020) and 2020–2021 (November 2020 to February 2021). In each sampled location, 30 ovitraps were installed in shaded areas. There was a predominance of the Aedes mosquitoes, especially Ae. albopictus and Ae. aegypti, while three species of Haemagogus (Hg. spegazzinii, Hg. janthinomys and Hg. leucocelaenus) were rarely found. The sylvatic mosquito Ae. terrens was abundant in areas with lower anthropic influence and during higher rainfall regimes with minimal pluviometric variation. This rainfall was also favorable for the presence of the predatory mosquito larvae Toxorhynchites theobaldi. The abundance of invasive Aedes species showed positive correlations with each other and negative correlations with the sylvatic Ae. terrens. Our results demonstrate that human occupation and the rainfall regime affect the interactions between invasive and sylvatic species of container mosquitoes.
1. Introduction
Globalization and urbanization are the main causes of the emergence and reemergence of significant diseases in the world, leading to a central concern to understand the role of insects as vectors in the transmission of diseases in humans and their distribution at different spatial scales [1,2]. The land use change from natural habitats to urban areas can negatively impact ecological processes (i.e., collapse of biotic interactions) as well as biodiversity, resulting in the loss of native species and the establishment and colonization of urban and peri-urban areas by opportunistic and invasive species [3,4,5]. For example, as a result of the urbanization process, many disease vector mosquitoes are able to increase their presence and distribution in urban and peri-urban areas, which has important implications for public health [6,7].
The distribution and occurrence of mosquitoes is affected by several factors and varies at different spatial scales [8]. Mosquito vector distribution is largely determined by climate at broad spatial scales [9]. At local and regional scales, such as in natural environments or peri-urban areas of municipalities, distribution and abundance patterns are affected by a multitude of biotic factors, including vegetation cover [10,11,12], host availability [13,14], and competition and predation interactions with other species [15,16]. Abiotic aspects such microclimate [17,18], availability of larval habitats [19,20], water temperature [21], conductivity and PH [22,23] have also impacted the abundance of vectors.
Invasive mosquito species of the genus Aedes such as Aedes aegypti and Ae. albopictus are the primary vectors of several human arboviruses, such as Dengue, Zika and Chikungunya [24]. These species are well established in Brazil and are excellent models for the study of vector dynamics in peri-urban areas [25,26,27]. Ae. aegypti are highly anthropophilic and prefer urban and suburban environments with high human population and residence density [28,29]. Comparatively, Ae. albopictus is found in vegetation-covered regions and areas with low-density human populations, but it has also been observed in transitional areas [30,31]. A variety of hosts has been already observed [32] and their eggs are drought resistant, remaining viable for months during dry season, and hatching when the rainy season starts [33]. Mosquitoes can occupy tree holes in natural environments or artificial containers in anthropogenic ones during the dry season, allowing them to resist drought [6].
Currently, there is great concern about the potential expansion and distribution of Ae. Albopictus into urban and peri-urban areas, which, in turn, would facilitate the arrival and colonization of pathogens that normally occur in natural habitats [34,35]. Although Ae. aegypti is considered the main vector of Dengue virus (DENV), Ae. albopictus can also contribute to the transmission of this disease in several regions around the world [36]. Beyond DENV, Ae. albopictus is also vector of the Chikungunya virus (CHIKV), which is responsible of recent outbreaks of this disease in France [37] and in countries of Central Africa [38]. During October 2019, transmission of the Zika virus (ZIKV) in France was also reported [39]. Recent studies have questioned the possible transmission of yellow fever and West Nile Virus by Ae. albopictus [34,40]. In Brazil, the infection of Ae. albopictus with DENV and ZIKV was reported for the first time during an outbreak in the rural zone of the coastal state of Espírito-Santo [41]. Hence, considering the species’ ability of colonizing natural, rural, peri-urban, and urban regions [42], it is considered a potential bridge vector between sylvatic (i.e., Yellow fever virus) and urban (i.e., DENV) arboviruses cycles [43], increasing the importance of detecting and monitoring its presence in different environments in Brazil.
Here, we tested whether the vector mosquito species differ in their spatial distribution considering three phytophysiognomies of a peri-urban area in Montes Claros city, north of the Minas Gerais state, Brazil. We also tested whether the pluviosity can influence the spatial distribution and abundance of mosquitoes. We also described the interspecific interactions between the native and invasive species.
2. Materials and Methods
Sampling was carried out in the municipality of Montes Claros, north of Minas Gerais. The climate classification is Aw (tropical–semi-arid), with high temperatures (annual average of 24.1 °C) and average annual rainfall of 1085 mm [44]. The study was conducted in a peri-urban area (16°45′31″ S 43°53′40″ W) located close to residential areas and formed by different vegetation types. The three phytophysiognomies where sampling was conducted correspond to a parcel of regenerating dry forest over limestone outcrops; a degraded Cerrado surrounded by an active subsistence pasture matrix (cattle and horses); and a riparian forest of a small watercourse. All areas are close to three residential neighborhoods, separated from them by a minimum distance of 400 m and a maximum distance of 1400 m.
We collected mosquitoes during two different rainy seasons. The first (2019–2020), occurred between October 2019 and February 2020. The second collection (2020–2021) was performed between November 2020 and February 2021. To capture immature mosquitoes, oviposition traps (ovitraps) were used [45]. Ovitraps are used as a standard for epidemiological surveillance as recommended by the WHO, especially in low infestations and when larval research proves to be unproductive [46]. For epidemiological surveillance, the ovitraps should preferably stay in the field for one week until the eggs are removed. In our case, the ovitraps were used as an artificial tree hole, modified from that proposed by Yanoviak and Fincke [47]. Before the beginning of each rainy season, in each sampled phytophysiognomy (dry forest, pasture, and riparian forest), 30 traps of the ovitrap type were arbitrarily installed in shaded or partially shaded places, totaling 90 traps in each rainy season. Each trap was numbered and tied to trees at breast height with a minimum distance of 10 m from each other. The traps were installed empty and remained in the field during the rainy season, naturally receiving the input of water and resources that accumulated during the trap’s exposure period. After 30 days of the first event of rain, 10 ovitraps were collected in each sampling area. Then, this procedure was repeated at intervals of 30 days until all traps were collected, totaling three mosquito collections in the 2019/2020 rainy season. In the 2020/2021 rainy season, the rain interruption allowed only two mosquito collections to be carried out. After each sampling, the traps were taken to the Laboratory of Ecology and Biological Control of Insects at the State University of Montes Claros, to rear the mosquitoes. The water collected from each trap was stored in an insectarium with controlled temperature (27 ± 2 °C) and light conditions (12 h dark/light). The original volume of the ovitraps was maintained by replacing the water with distilled water in each trap. The larvae were inspected daily until pupae were obtained, which were then transferred to 100 mL plastic cups and placed in a hatching trap to allow the adult mosquitoes to emerge. Mosquito species were identified at the species level using identification keys proposed by Forattini (2002) and Consoli and Oliveira-Filho (1994). To obtain adult mosquitoes of the Toxorhynchites species, predatory larvae were fed with larvae of other species collected from the study areas. Rainfall data were obtained from the online platform of the National Institute of Meteorology (INMET).
Generalized linear models (GLMs) were constructed to test the effects of biotic (interaction between mosquito species) and abiotic factors (sampled sites and rainfall regimes) (i.e., explanatory variables) on mosquito abundance (response variable). The rainfall regime was assessed based on the total rainfall during the sampling periods and the accumulated rainfall in the 14 days preceding the removal of the ovitraps from the field. These time intervals were chosen to ensure adequate time for the complete development of mosquitoes. The complete models were reduced through a stepwise procedure, and the results were subjected to analysis of variance (ANOVA), considering p-values < 0.05 as statistically significant. Contrast analysis was performed to assess the significant differences among the study sites. Residual analysis was conducted to examine the homogeneity of variance and the adequacy of error distribution using the diagnostic function of the ‘RT4Bio’ package. Analyses were performed using statistical software R (R Development Core Team, 2015).
3. Results
A total of 2689 adult mosquitoes were obtained in all study sites, grouped into two subfamilies (Culicinae and Toxorhynchitinae), three genera, and seven species (Table 1). There was a predominance of mosquitoes of the genus Aedes, which corresponded to 97.73% of the total number of sampled mosquitoes (Table 1).

Table 1.
Total and relative abundance of mosquito species sampled in three peri-urban phytophysiognomies of Montes Claros, MG, during the rainy seasons of 2019–2020 and 2020–2021.
3.1. Influence of Sampled Sites and Habitat Characteristics
The presence of Ae. aegypti, Ae. albopictus, and Tx. theobaldi was detected in all sampling areas. Alternatively, Hg. leucocelaenus and Hg. janthinomys were found only in the Riparian forest. There was a significant variation in the abundance of Ae. albopictus (deviance = 17.597; p < 0.05) through the phytophysiognomies studied. However, sampling sites did not influence the abundance of Ae. aegypti and Ae. terrens.
3.2. Rainfall Regime
The abundance of Ae. albopictus did not exhibit a significant relationship between rainfall variation and the accumulated rainfall 14 days before sampling. However, the abundance of Ae. aegypti showed a significant relationship with the interaction between rainfall variation and the accumulated rainfall 14 days before sampling (deviance = 4.7331, p < 0.05). The highest densities were observed in rainfall regimes of slight variation and intermediate accumulated volumes in the riparian and dry forest. In the pasture area, the highest densities were found in regimes with slight variation, but relatively small accumulated volumes in the previous 14 days (Figure 1). The abundance of Ae. terrens showed a significant relationship with rainfall variation × accumulated rainfall 14 days before sampling in all analyzed environments (deviance = 26.818, p < 0.05). The results indicate that the highest abundances of Ae. terrens were associated with the interaction between the low volumes of rainfall accumulated during the 14 days prior to sampling, particularly in regimes of high variance in the riparian forest and pasture areas. On the other hand, in the dry forest, the highest abundances were observed when there was an interaction between regimes with higher accumulated volumes and lower variance (Figure 2). The abundance of Tx. theobaldi was found to be related to the total volume of accumulated rainfall (deviance = 11.0903, p < 0.05) and with the variation of the rainfall regime (deviance = 8.7467, p < 0.05) (Figure 3), not differing in abundance between the sampled areas.
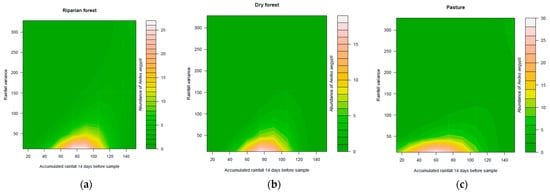
Figure 1.
Effect of rainfall variation and accumulated precipitation on the abundance of Aedes aegypti in three peri-urban environments ((a)—riparian forest, (b)—dry forest, and (c)—pasture) in the city of Montes Claros, northern region of Minas Gerais state, Brazil.
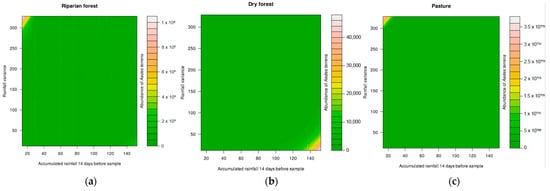
Figure 2.
Effect of rainfall variation and accumulated precipitation on the abundance of Aedes terrens in three peri-urban environments ((a)—riparian forest, (b)—dry forest, and (c)—pasture) in the city of Montes Claros, northern region of Minas Gerais state, Brazil.
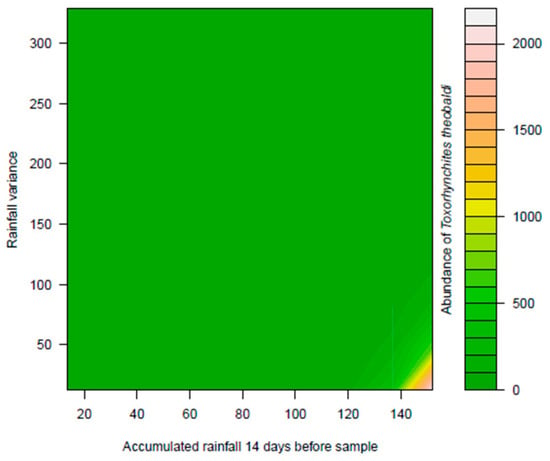
Figure 3.
Effect of rainfall variation and accumulated precipitation on the abundance of Toxorhynchites theobaldi in peri-urban environments in the city of Montes Claros, northern region of Minas Gerais state, Brazil.
3.3. Interaction between Species
The abundance of Ae. albopictus was positively correlated with the abundance of Ae. aegypti (Y = e(2.31 + 031 × LogX))(deviance = 9.3901, p < 0.05) (Figure 4). In contrast, the abundance of Ae. albopictus in each trap did not vary with the abundance of Ae. terrens. The abundance of Ae. aegypti in each trap was negatively correlated with the abundance of the native mosquito Ae. terrens (Y = e(1.32 − 0.54logX)) (deviance = 4.6207, p < 0.05) (Figure 5). The abundance of Tx. theobaldi in each trap was unrelated to the abundance of any other mosquito in this study.
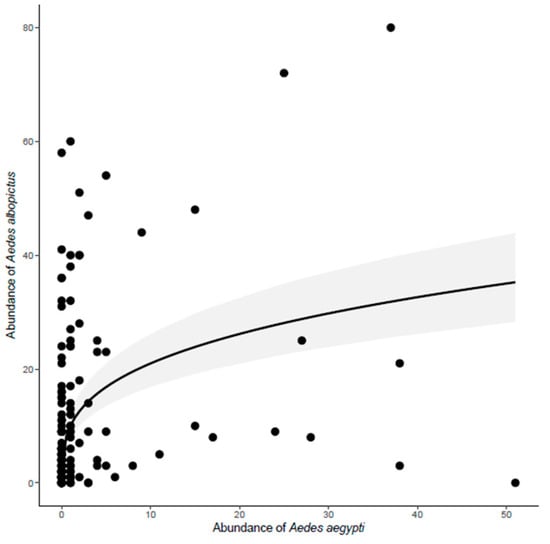
Figure 4.
Effect of Aedes albopictus abundance on the variation in Ae. aegypti abundance, sampled in ovitraps distributed in three peri-urban environments in the city of Montes Claros, northern region of Minas Gerais state, Brazil. Each point represents an individual ovitrap collected in the study area.
second round.
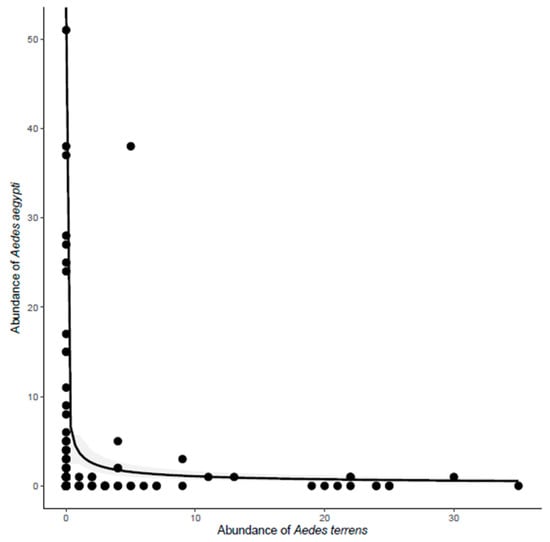
Figure 5.
Effect of Aedes aegypti abundance on the variation in Ae. terrens abundance, sampled in ovitraps distributed in three peri-urban environments in the city of Montes Claros, northern region of Minas Gerais state, Brazil. Each point represents an individual ovitrap collected in the study area.
4. Discussion
In this study, invasive Ae. albopictus and Ae. aegypti were found to be the predominant species among the three phytophysiognomies tested, followed by the native mosquito Ae. terrens and the predatory larvae mosquito Toxorhynchites theobaldi. Additionally, there was also the punctual presence of Hg. spegazzini and the wild YFV vectors, Hg. leucocelaenus and Hg. janthinomys. The observed low level of diversity can be attributed to the urban matrix surrounding the sampled sites, which is often associated with low species richness and diversity in tropical areas [48,49]. The region of northern Minas Gerais also exhibits strong seasonality, characterized by distinct rainy and dry seasons with extended periods of drought lasting more than six months [50]. Due to the semi-arid climate and prolonged intervals between rainfall, oviposition sites may be less productive, resulting in decreased abundance and diversity among container-breeding species [51,52]. In strongly seasonal environments like these, the presence of urban areas tends to favor the establishment of invasive species, which are able to survive in artificial containers within cities, although with reduced abundance during dry seasons.
Habitat characteristics are often described as a determining factor in spatial distribution studies of Aedes species [53,54]. In this study, Ae. albopictus was found to be predominant in all sampled locations throughout both years of the study. This finding is consistent with the widely accepted view that this mosquito is more abundant in peri-urban and wild areas [28,55]. Recent studies have provided evidence that the food preference patterns of Ae. albopictus can be more accurately classified as anthropophagy and opportunism rather than anthropophilia [32,43]. As a result of this behavior, Ae. albopictus is more likely to transmit zoonotic pathogens in environments where humans are present at intermediate levels, such as peri-urban areas and transition areas [43,56]. Several studies suggest that peri-domestic mosquitoes, like Ae. malayensis, escape vector control measures targeting domestic vector species and may contribute to the reemergence of arbovirus [57]. Hence, the comparable abundance of Ae. albopictus in both pasture and dry forest areas could be attributed to the presence of hosts such as horses and cattle, as well as the availability of the traps as breeding sites.
Despite the well-known highly anthropophilic behavior of Ae. aegypti [28,55,58], our study revealed the presence of this mosquito species in all sampled sites within the peri-urban environment. The occurrence of Ae. aegypti has also been reported in forest parks within Porto Alegre, in an Atlantic Forest fragment located far from the edge of the forest [59]. This mosquito was also found 100 m inside urban parks in Manaus, including areas with dense vegetation that are inaccessible to the public [60], highlighting the ability of this species to inhabit different environments. Hence, our study demonstrates that this invasive mosquito coexists with native and transitional species in peri-urban areas. Despite the lower abundance compared to that of Ae. albopictus, the presence of Ae. aegypti raises concerns about transmission of urban arboviruses in peri-urban environments and should be monitored.
The abundance patterns of mosquitoes in these peri-urban areas may have also been influenced by rainfall in the days prior to sampling. Aedes species are known to colonize temporary breeding sites [61], and outbreaks of diseases transmitted by these mosquitoes regularly coincide with the rainy seasons [62,63]. The abundance of Ae. aegypti did not differ between the sampled sites, which suggests that during the rainy season, this mosquito increases its densities in urban environments where there is greater availability of human hosts, absence of predators and natural competitors, and the ability to reproduce in artificial containers [64]. Thus, denser populations on the edges of cities favor the penetration of individuals in peri-urban areas. The abundance of Ae. aegypti was higher in rainfall regimes with slight variation in the 14 days preceding the collections, as well as in rainfall regimes of small/intermediate intensity and greater amplitude in the pasture area. This species often occurs in areas with suboptimal environmental conditions, and Ae. aegypti females exhibit opportunistic laying behavior, distributing their eggs among several breeding sites and being undemanding regarding the biotic conditions of these breeding places [33]. Furthermore, the eggs are resistant to desiccation and most of them are deposited individually on the reservoir wall, just above the water surface [25]. However, some eggs (varying from 4% to 62%) can also be deposited directly on the water surface, which helps to maintain the mosquito population in the dry season, when there is no new water input [65,66]. Such conditions, therefore, facilitate the establishment of this species even in regions with low rainfall.
The relationship between the abundance of Ae. terrens and rainfall variables indicates a strong relationship with the dry forest, which is associated with high intensity of rainfall with minimal variation. In contrast, in areas closer to human activities such as the pasture and the riparian forest, the presence of Ae. terrens occasionally occurs under conditions of high rainfall variability, particularly during the peak of the rainfall. This species has a wide geographic distribution, recorded from the south of the Amazon basin in Brazil to the northern region of Argentina and in several countries of South America [67]. Despite the limited literature, experimental studies have described this mosquito as a possible vector of arboviruses. An experimental study found that populations of Ae. terrens were competent in transmitting both lineages of the CHIKV circulating in the Americas [68].
The same pattern can be observed for the predatory mosquito Tx. theobaldi. Our findings agree with those of other studies that suggest that wild species are typically less prone to adaptation [69] and are influenced by the volume of water retained in the containers and the stability of rainfall in these environments. Females of the genus Toxorhynchites lay their eggs individually on the water’s surface in both natural and artificial containers [70,71]. Additionally, females of this species disperse their eggs widely to minimize the risk of cannibalism among the offspring and ensure the availability of prey resources [72]. Therefore, this oviposition behavior may be associated with the low densities of Tx. theobaldi observed in all sampled sites. Species belonging to this genus exhibit predatory behavior during their larval stages and have been extensively evaluated for their potential role as biological control agents [73].
The establishment of invasive species is related to the characteristics of the invader, the environment, and the resident community [74,75]. The consequences of competitive interactions between species of the genus Aedes depend on both the environment and on the level of urbanization in the surrounding areas [31]. The density of Ae. albopictus does not vary in relation to the densities of native species, Ae. terrens and Tx. theobaldi. The mosquito Ae. albopictus is native to Southeast Asia [76,77] and is considered one of the most widespread invasive species worldwide [78,79,80]. Although the competitive superiority of Ae. albopictus over Ae. aegypti has been observed in field and laboratory experiments [81], the effects of interspecific competition were not enough to displace Ae. aegypti in the study areas. In this scenario, our findings corroborate the idea that in peri-urban or transitional areas Ae. aegypti and Ae. albopictus coexist in large numbers [28,55]. Furthermore, the differential effects of interactions between invasive species and the native community may promote coexistence.
The abundance of the Ae. aegypti mosquito decreases with the presence of the native mosquito Ae. terrens. Therefore, despite the spillover of Ae. aegypti from urban to peri-urban areas in drier regimes, the conditions and adaptation of native Ae. terrens may have buffered the persistence of Ae. aegypti in these locations. A similar effect has been observed in urban neighborhoods of Baltimore, MD, USA, where competitive interactions between the resident mosquito Culex pipiens, the primary vector of West Nile virus, and Ae. albopictus depend on specific interaction conditions and the types of containers, thereby facilitating the persistence of Cx. pipiens despite the invasion of Ae. albopictus [82]. Recently, studies have demonstrated that Ae. terrens can be infected with the Guapiaçu virus, a newly identified virus phylogenetically similar to host-related insect-specific flavivirus (dISFV) [83]. Thus, the possibility of host switching exists, as reported with other arboviruses.
Overall, our study shows that complex interactions between abiotic and biotic factors determine distribution patterns and abundance of mosquito species in peri-urban areas. Our findings suggest that in peri-urban environments within strongly seasonal regions, the buffering effect exerted by native biota on the spillover of invasive species, typically associated with urban areas, is relatively weaker. This effect is primarily attributed to the drastic reduction in wild mosquito densities during the dry season, which, in turn, facilitates the local colonization by typically urban species, at least at the beginning of the rainy season. Consequently, in seasonal environments, invasive mosquito species coexist with native species. This overlapping of distributions can facilitate the flow of diseases and pathogens from wild to urban environments and vice versa. Therefore, our research demonstrates the importance of monitoring ecotonal areas as a central aspect of health surveillance measures.
Author Contributions
Conceptualization, H.R.-S., R.R.-J., P.H.C.C., M.L.F. and M.A.Z.B.; methodology, H.R.-S., R.R.-J., P.H.C.C., M.L.F. and M.A.Z.B.; material collection, H.R.-S., J.G.C., P.H.C.C., M.L.F. and M.A.Z.B.; formal analysis, H.R.-S., R.R.-J., M.L.F., P.C.-R. and M.A.Z.B.; investigation, H.R.-S., J.G.C., R.R.-J., P.H.C.C., M.L.F., S.P.R., F.V.S.A., P.C.-R. and M.A.Z.B.; resources, H.R.-S. and M.A.Z.B.; writing—original draft preparation, H.R.-S., M.L.F., S.P.R., F.V.S.A., P.C.-R. and M.A.Z.B.; writing—review and editing, H.R.-S., J.G.C., R.R.-J., P.H.C.C., M.L.F., S.P.R., F.V.S.A., P.C.-R. and M.A.Z.B.; visualization, H.R.-S., J.G.C., R.R.-J., P.H.C.C., M.L.F., S.P.R., F.V.S.A., P.C.-R. and M.A.Z.B.; supervision, H.R.-S., P.H.C.C., M.L.F., S.P.R., F.V.S.A., P.C.-R. and M.A.Z.B.; project administration, H.R.-S., F.V.S.A. and M.A.Z.B.; funding acquisition, H.R-S. and M.A.Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COORDENAÇÃO DE APRIMORAMENTO DE PESSOAL DE NÍVEL SUPERIOR (CAPES), grant number 88882.436098/2019-01.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to all institutions and technicians who contributed to and supported the fieldwork. Special thanks are extended to Alice Meiry Silva Dias and Luana dos Reis Fiúza for their invaluable contributions during the fieldwork and mosquito identification. Additionally, we would like to thank the residents of the collection sites for their willingness to assist the researchers. We are also grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for awarding the scholarship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Beier, J.C.; Benelli, G. Complexity of the Relationship between Global Warming and Urbanization—An Obscure Future for Predicting Increases in Vector-Borne Infectious Diseases. Curr. Opin. Insect Sci. 2019, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Munshi-South, J. Evolution of Life in Urban Environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef] [PubMed]
- Knop, E. Biotic Homogenization of Three Insect Groups due to Urbanization. Glob. Chang. Biol. 2016, 22, 228–236. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.-P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr. Biol. 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Chandrasegaran, K.; Lahondère, C.; Escobar, L.E.; Vinauger, C. Linking Mosquito Ecology, Traits, Behavior, and Disease Transmission. Trends Parasitol. 2020, 36, 393–403. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.-M. Global Distribution of Aedes Aegypti and Aedes Albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Gardner, A.M.; Anderson, T.K.; Hamer, G.L.; Johnson, D.E.; Varela, K.E.; Walker, E.D.; Ruiz, M.O. Terrestrial Vegetation and Aquatic Chemistry Influence Larval Mosquito Abundance in Catch Basins, Chicago, USA. Parasites Vectors 2013, 6, 9. [Google Scholar] [CrossRef]
- Wimberly, M.C.; Davis, J.K.; Evans, M.V.; Hess, A.; Newberry, P.M.; Solano-Asamoah, N.; Murdock, C.C. Land Cover Affects Microclimate and Temperature Suitability for Arbovirus Transmission in an Urban Landscape. PLoS Negl. Trop. Dis. 2020, 14, e0008614. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Turo, K.J.; Riley, C.B.; Inocente, E.A.; Tian, J.; Hoekstra, N.C.; Piermarini, P.M.; Gardiner, M.M. Can Urban Greening Increase Vector Abundance in Cities? The Impact of Mowing, Local Vegetation, and Landscape Composition on Adult Mosquito Populations. Urban Ecosyst. 2019, 22, 827–839. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; McClure, C.J.W.; Estep, L.K.; Eubanks, M.D. Hosts or Habitats: What Drives the Spatial Distribution of Mosquitoes? Ecosphere 2013, 4, 30. [Google Scholar] [CrossRef]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes Albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl. Trop. Dis. 2014, 8, e3037. [Google Scholar] [CrossRef]
- Fader, J.E. The Importance of Interspecific Interactions on the Present Range of the Invasive Mosquito Aedes Albopictus (Diptera: Culicidae) and Persistence of Resident Container Species in the United States. J. Med. Entomol. 2016, 53, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; Westby, K.M.; Ower, G.D. Know Your Enemy: Effects of a Predator on Native and Invasive Container Mosquitoes. J. Med. Entomol. 2019, 56, 320–328. [Google Scholar] [CrossRef]
- Lounibos, L.P.; O’Meara, G.F.; Juliano, S.A.; Nishimura, N.; Escher, R.L.; Reiskind, M.H.; Cutwa, M.; Greene, K. Differential Survivorship of Invasive Mosquito Species in South Florida Cemeteries: Do Site-Specific Microclimates Explain Patterns of Coexistence and Exclusion? Ann. Entomol. Soc. Am. 2010, 103, 757–770. [Google Scholar] [CrossRef]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-Scale Variation in Microclimate across an Urban Landscape Shapes Variation in Mosquito Population Dynamics and the Potential of Aedes Albopictus to Transmit Arboviral Disease. PLoS Negl. Trop. Dis. 2017, 11, e0005640. [Google Scholar] [CrossRef]
- Evans, M.V.; Hintz, C.W.; Jones, L.; Shiau, J.; Solano, N.; Drake, J.M.; Murdock, C.C. Microclimate and Larval Habitat Density Predict Adult Aedes Albopictus Abundance in Urban Areas. Am. J. Trop. Med. Hyg. 2019, 101, 362–370. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Medina, J.; Chase, C.; Cardenas, G.; Mutebi, J.-P.; Petrie, W.D.; Beier, J.C. Proliferation of Aedes Aegypti in Urban Environments Mediated by the Availability of Key Aquatic Habitats. Sci. Rep. 2020, 10, 12925. [Google Scholar] [CrossRef]
- Krol, L.; Gorsich, E.E.; Hunting, E.R.; Govender, D.; van Bodegom, P.M.; Schrama, M. Eutrophication Governs Predator-Prey Interactions and Temperature Effects in Aedes Aegypti Populations. Parasites Vectors 2019, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Sousa, A.R.; de Oliveira-Christe, R.; Camargo, A.A.; Scinachi, C.A.; Milani, G.M.; Urbinatti, P.R.; Natal, D.; Ceretti-Junior, W.; Marrelli, M.T. Influence of Water’s Physical and Chemical Parameters on Mosquito (Diptera: Culicidae) Assemblages in Larval Habitats in Urban Parks of São Paulo, Brazil. Acta Trop. 2020, 205, 105394. [Google Scholar] [CrossRef] [PubMed]
- Kinga, H.; Kengne-Ouafo, J.A.; King, S.A.; Egyirifa, R.K.; Aboagye-Antwi, F.; Akorli, J. Water Physicochemical Parameters and Microbial Composition Distinguish Anopheles and Culex Mosquito Breeding Sites: Potential as Ecological Markers for Larval Source Surveillance. J. Med. Entomol. 2022, 59, 1817–1826. [Google Scholar] [CrossRef]
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; RADAM-LAC Research Team; Talbot, B. Arbovirus Vectors of Epidemiological Concern in the Americas: A Scoping Review of Entomological Studies on Zika, Dengue and Chikungunya Virus Vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef]
- Carvalho, F.D.; Moreira, L.A. Why Is Aedes Aegypti Linnaeus so Successful as a Species? Neotrop. Entomol. 2017, 46, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the Global Potential Distributions of Two Arboviral Vectors Aedes Aegypti and Ae. Albopictus under Changing Climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [PubMed]
- Kotsakiozi, P.; Gloria-Soria, A.; Caccone, A.; Evans, B.; Schama, R.; Martins, A.J.; Powell, J.R. Tracking the Return of Aedes Aegypti to Brazil, the Major Vector of the Dengue, Chikungunya and Zika Viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005653. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourençqo-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef]
- Benitez, E.M.; Ludueña-Almeida, F.; Frías-Céspedes, M.; Almirón, W.R.; Estallo, E.L. Could Land Cover Influence Aedes Aegypti Mosquito Populations? Med. Vet. Entomol. 2019, 34, 138–144. [Google Scholar] [CrossRef]
- Leisnham, P.T.; LaDeau, S.L.; Juliano, S.A. Spatial and Temporal Habitat Segregation of Mosquitoes in Urban Florida. PLoS ONE 2014, 9, e91655. [Google Scholar] [CrossRef]
- Hopperstad, K.A.; Sallam, M.F.; Reiskind, M.H. Estimations of Fine-Scale Species Distributions of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) in Eastern Florida. J. Med. Entomol. 2021, 58, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Fikrig, K.; Harrington, L.C. Understanding and Interpreting Mosquito Blood Feeding Studies: The Case of Aedes Albopictus. Trends Parasitol. 2021, 37, 959–975. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes Aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.d.A.; Santos, F.B.D.; Vazeille, M.; Vasconcelos, P.F.d.C.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Potential Risk of Re-Emergence of Urban Transmission of Yellow Fever Virus in Brazil Facilitated by Competent Aedes Populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lima-Camara, T.N. The Asian Tiger Mosquito in Brazil: Observations on Biology and Ecological Interactions since Its First Detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes Albopictus, an Arbovirus Vector: From the Darkness to the Light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’Ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferre, J.B.; Catelinois, O.; et al. Chikungunya Outbreak in Montpellier, France, September to October 2014. Eurosurveillance 2015, 20, 21108. [Google Scholar] [CrossRef]
- Paupy, C.; Kassa Kassa, F.; Caron, M.; Nkoghé, D.; Leroy, E.M. A Chikungunya Outbreak Associated with the Vector Aedes Albopictus in Remote Villages of Gabon. Vector-Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The First Local Cases of Zika Virus in Europe. Lancet 2019, 394, 1991–1992. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Ricas Rezende, H.; Malta Romano, C.; Morales Claro, I.; Santos Caleiro, G.; Cerdeira Sabino, E.; Felix, A.C.; Bissoli, J.; Hill, S.; Rodrigues Faria, N.; Cardoso da Silva, T.C.; et al. First Report of Aedes Albopictus Infected by Dengue and Zika Virus in a Rural Outbreak in Brazil. PLoS ONE 2020, 15, e0229847. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A Systematic Review: Is Aedes Albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Dos-Santos, T.; Roiz, D.; Abreu, F.V.S.; Luz, S.L.B.; Santalucia, M.; Jiolle, D.; Santos Neves, M.S.A.; Simard, F.; Lourenço-de-Oliveira, R.; Paupy, C. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg. Microbes Infect. 2018, 7, 191. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- Eliason, D.A. A Preferred Oviposition Site as a Surveillance Method for Aedes Aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Yanoviak, S.P.; Fincke, O.M. Sampling Methods for Water-Filled Tree Holes and Their Artificial Analogues. In Insect Sampling in Forest Ecosystems; Leather, S.R., Ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 168–185. ISBN 978047075051348. [Google Scholar]
- Perfecto, I.; Vandermeer, J. Biodiversity Conservation in Tropical Agroecosystems: A New Conservation Paradigm. Ann. N. Y. Acad. Sci. 2008, 1134, 173–200. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Benelli, G.; Beier, J.C. Anthropogenic Changes and Associated Impacts on Vector-Borne Diseases. Trends Parasitol. 2021, 37, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Souza, D.; Oliveira, F.G.; de Castro, I.L.; de Souza Soares, J.B.; Reis, M.M.; de Figueiredo, F.P. Frequência de ocorrência de precipitação pluviométrica em Montes Claros-MG. Agrarian 2018, 11, 337–342. [Google Scholar] [CrossRef]
- Petermann, J.S.; Gossner, M.M. Aquatic Islands in the Sky: 100 Years of Research on Water-Filled Tree Holes. Ecol. Evol. 2022, 12, e9206. [Google Scholar] [CrossRef]
- Ptatscheck, C.; Traunspurger, W. Meio- and Macrofaunal Communities in Artificial Water-Filled Tree Holes: Effects of Seasonality, Physical and Chemical Parameters, and Availability of Food Resources. PLoS ONE 2015, 10, e0133447. [Google Scholar] [CrossRef]
- Bennett, K.L.; McMillan, W.O.; Enríquez, V.; Barraza, E.; Díaz, M.; Baca, B.; Whiteman, A.; Cerro Medina, J.; Ducasa, M.; Gómez Martínez, C.; et al. The Role of Heterogenous Environmental Conditions in Shaping the Spatiotemporal Distribution of Competing Aedes Mosquitoes in Panama: Implications for the Landscape of Arboviral Disease Transmission. Biol. Invasions 2021, 23, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Friberg, M.D. Aedes Albopictus and Aedes Flavopictus (Diptera: Culicidae) Pre-Imaginal Abundance Patterns Are Associated with Different Environmental Factors along an Altitudinal Gradient. Curr. Res. Insect Sci. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Honório, N.A.; Castro, M.G.; Barros, F.S.M.d.; Magalhães, M.d.A.F.M.; Sabroza, P.C. The Spatial Distribution of Aedes Aegypti and Aedes Albopictus in a Transition Zone, Rio de Janeiro, Brazil. Cad. Saúde Pública 2009, 25, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Fikrig, K.; Rose, N.; Burkett-Cadena, N.; Kamgang, B.; Leisnham, P.T.; Mangan, J.; Ponlawat, A.; Rothman, S.E.; Stenn, T.; McBride, C.S.; et al. Aedes Albopictus Host Odor Preference Does Not Drive Observed Variation in Feeding Patterns across Field Populations. Sci. Rep. 2023, 13, 130. [Google Scholar] [CrossRef]
- Mendenhall, I.H.; Manuel, M.; Moorthy, M.; Lee, T.T.M.; Low, D.H.W.; Missé, D.; Gubler, D.J.; Ellis, B.R.; Ooi, E.E.; Pompon, J. Peridomestic Aedes Malayensis and Aedes Albopictus Are Capable Vectors of Arboviruses in Cities. PLoS Negl. Trop. Dis. 2017, 11, e0005667. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Kramer, L.D. Invasiveness of Aedes Aegypti and Aedes Albopictus and Vectorial Capacity for Chikungunya Virus. J. Infect. Dis. 2016, 214, S453–S458. [Google Scholar] [CrossRef]
- Montagner, F.R.G.; Silva, O.S.; Jahnke, S.M. Mosquito Species Occurrence in Association with Landscape Composition in Green Urban Areas. Braz. J. Biol. 2018, 78, 233–239. [Google Scholar] [CrossRef]
- Hendy, A.; Hernandez-Acosta, E.; Chaves, B.A.; Fé, N.F.; Valério, D.; Mendonça, C.; Lacerda, M.V.G.d.; Buenemann, M.; Vasilakis, N.; Hanley, K.A. Into the Woods: Changes in Mosquito Community Composition and Presence of Key Vectors at Increasing Distances from the Urban Edge in Urban Forest Parks in Manaus, Brazil. Acta Trop. 2020, 206, 105441. [Google Scholar] [CrossRef]
- Soghigian, J.; Andreadis, T.G.; Livdahl, T.P. From Ground Pools to Treeholes: Convergent Evolution of Habitat and Phenotype in Aedes Mosquitoes. BMC Evol. Biol. 2017, 17, 262. [Google Scholar] [CrossRef]
- Viana, D.V.; Ignotti, E. The Ocurrence of Dengue and Weather Changes in Brazil: A Systematic Review. Rev. Bras. Epidemiol. 2013, 16, 240–256. [Google Scholar] [CrossRef]
- Valdez, L.D.; Sibona, G.J.; Condat, C.A. Impact of Rainfall on Aedes Aegypti Populations. Ecol. Model. 2018, 385, 96–105. [Google Scholar] [CrossRef]
- Talaga, S.; Dejean, A.; Azémar, F.; Dumont, Y.; Leroy, C. Impacts of Biotic and Abiotic Parameters on Immature Populations of Aedes Aegypti. J. Pest Sci. 2020, 93, 941–952. [Google Scholar] [CrossRef]
- Soares, F.A.; Silva, J.C.; Oliveira, J.B.B.S.; Abreu, F.V.S. Study of oviposition behavior of Aedes aegypti in two neighborhoods under the influence of semi-arid climate in the municipality of Salinas, State of Minas Gerais, Brazil. Rev. Patol. Trop. 2015, 44, 77–88. [Google Scholar]
- Madeira, N.G.; Macharelli, C.A.; Carvalho, L.R. Variation of the oviposition preferences of Aedes aegypti in function of substratum and humidity. Memórias Inst. Oswaldo Cruz 2002, 97, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Schick, R.X. Mosquito Studies (Diptera, Culicidae) XX. The Terrens Group of Aedes (Finlaya). Contrib. Am. Entomol. Inst. 1970, 5, 1–158. [Google Scholar]
- Lourenço-de-Oliveira, R.; Failloux, A.-B. High Risk for Chikungunya Virus to Initiate an Enzootic Sylvatic Cycle in the Tropical Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005698. [Google Scholar] [CrossRef]
- Silva, S.O.F.; de Mello, C.F.; Julião, G.R.; Dias, R.; Alencar, J. Sexual Proportion and Egg Hatching of Vector Mosquitos in an Atlantic Forest Fragment in Rio de Janeiro, Brazil. Life 2022, 13, 13. [Google Scholar] [CrossRef]
- Trimble, R.M. Laboratory Observations on Oviposition by the Predaceous Tree-Hole Mosquito, Toxorhynchites Rutilus Septentrionalis (Diptera: Culicidae). Can. J. Zool. 1979, 57, 1104–1108. [Google Scholar] [CrossRef]
- Donald, C.L.; Siriyasatien, P.; Kohl, A. Toxorhynchites Species: A Review of Current Knowledge. Insects 2020, 11, 747. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Dame, D.A.; Bailey, D.L. Ability of Toxorhynchites Amboinensis (Doleschall) (Diptera: Culicidae) to Locate and Oviposit in Artificial Containers in an Urban Environment. Environ. Entomol. 1983, 12, 1073–1077. [Google Scholar] [CrossRef]
- Focks, D.A. Toxorhynchites as Biocontrol Agents. J. Am. Mosq. Control. Assoc. 2007, 23, 118–127. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by Insect Vectors of Human Disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef]
- Giunti, G.; Becker, N.; Benelli, G. Invasive Mosquito Vectors in Europe: From Bioecology to Surveillance and Management. Acta Trop. 2023, 239, 106832. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The Invasive Mosquito Species Aedes Albopictus: Current Knowledge and Future Perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, V.; Agostini, V.; Moroni, E.; Colombo, G.; Lombardo, G.; Rambaldi Migliore, N.; Gabrieli, P.; Garofalo, M.; Gagliardi, S.; Gomulski, L.M.; et al. The Worldwide Spread of Aedes Albopictus: New Insights from Mitogenomes. Front. Genet. 2022, 13, 931163. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the Tiger: Global Risk of Invasion by the Mosquito Aedes Albopictus. Vector-Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes Aegypti and Aedes Albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.G.; Lourenço-de-Oliveira, R.; Braga, I.A. Updating the Geographical Distribution and Frequency of Aedes Albopictus in Brazil with Remarks Regarding Its Range in the Americas. Memórias Inst. Oswaldo Cruz 2014, 109, 787–796. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Juliano, S.A. Where Vectors Collide: The Importance of Mechanisms Shaping the Realized Niche for Modeling Ranges of Invasive Aedes Mosquitoes. Biol. Invasions 2018, 20, 1913–1929. [Google Scholar] [CrossRef]
- Leisnham, P.T.; LaDeau, S.L.; Saunders, M.E.M.; Villena, O.C. Condition-Specific Competitive Effects of the Invasive Mosquito Aedes Albopictus on the Resident Culex Pipiens among Different Urban Container Habitats May Explain Their Coexistence in the Field. Insects 2021, 12, 993. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, G.; da Costa, A.C.; Gill, D.E.; Ribeiro, E.S.D.; da S. Rego, M.O.; Monteiro, F.J.C.; Villanova, F.; Nogueira, J.S.; Maeda, A.Y.; de Souza, R.P.; et al. Guapiaçu Virus, a New Insect-Specific Flavivirus Isolated from Two Species of Aedes Mosquitoes from Brazil. Sci. Rep. 2021, 11, 4674. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).