Shallow Hard-Bottom Benthic Assemblages of South Bay (Antarctic Peninsula): An Update 40 Years Later
Abstract
:1. Introduction
2. Materials and Methods
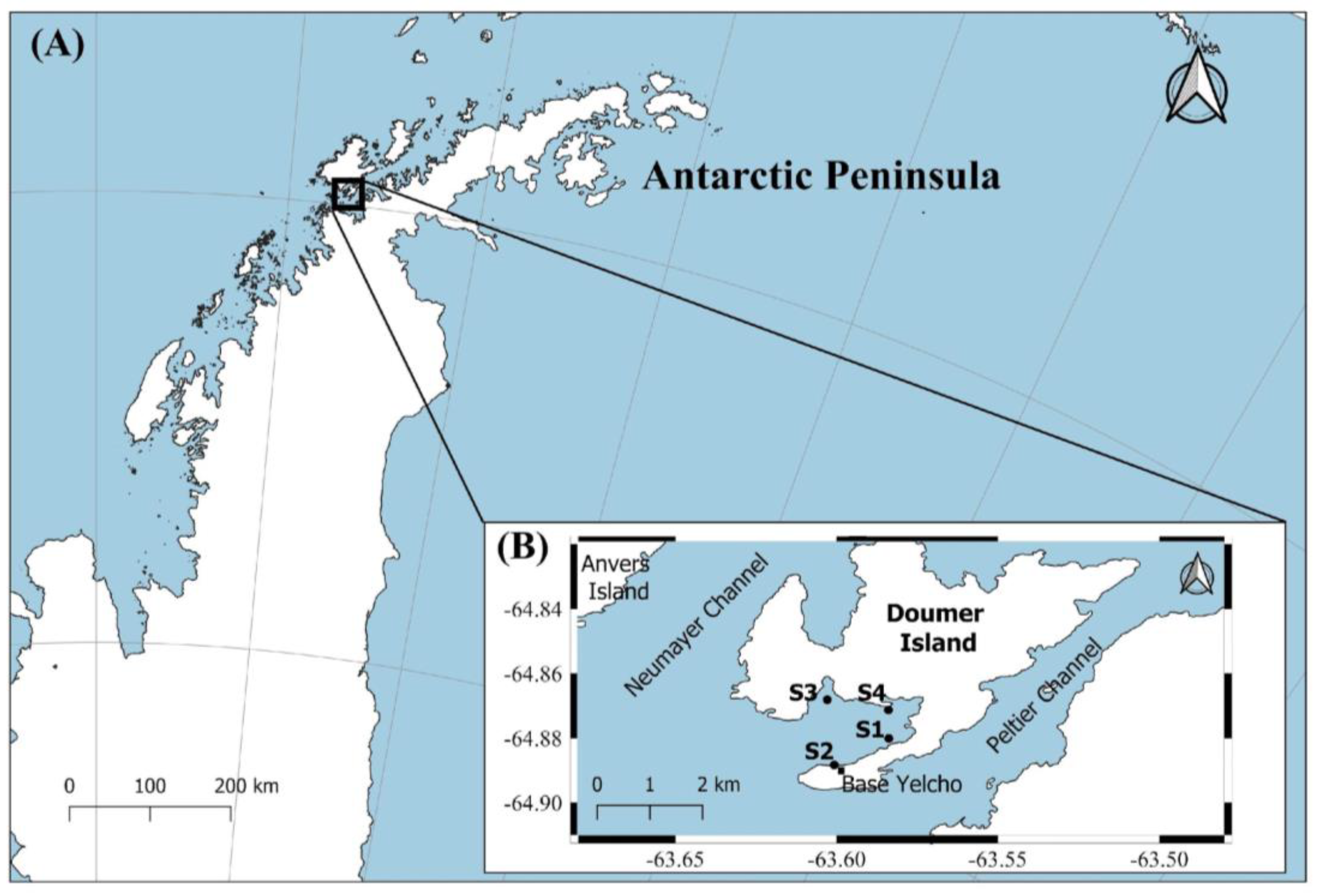
2.1. Study Area
2.2. Sampling Design and Data Analysis
3. Results
3.1. Taxonomic Diversity
3.2. Percentage Coverage and Densities
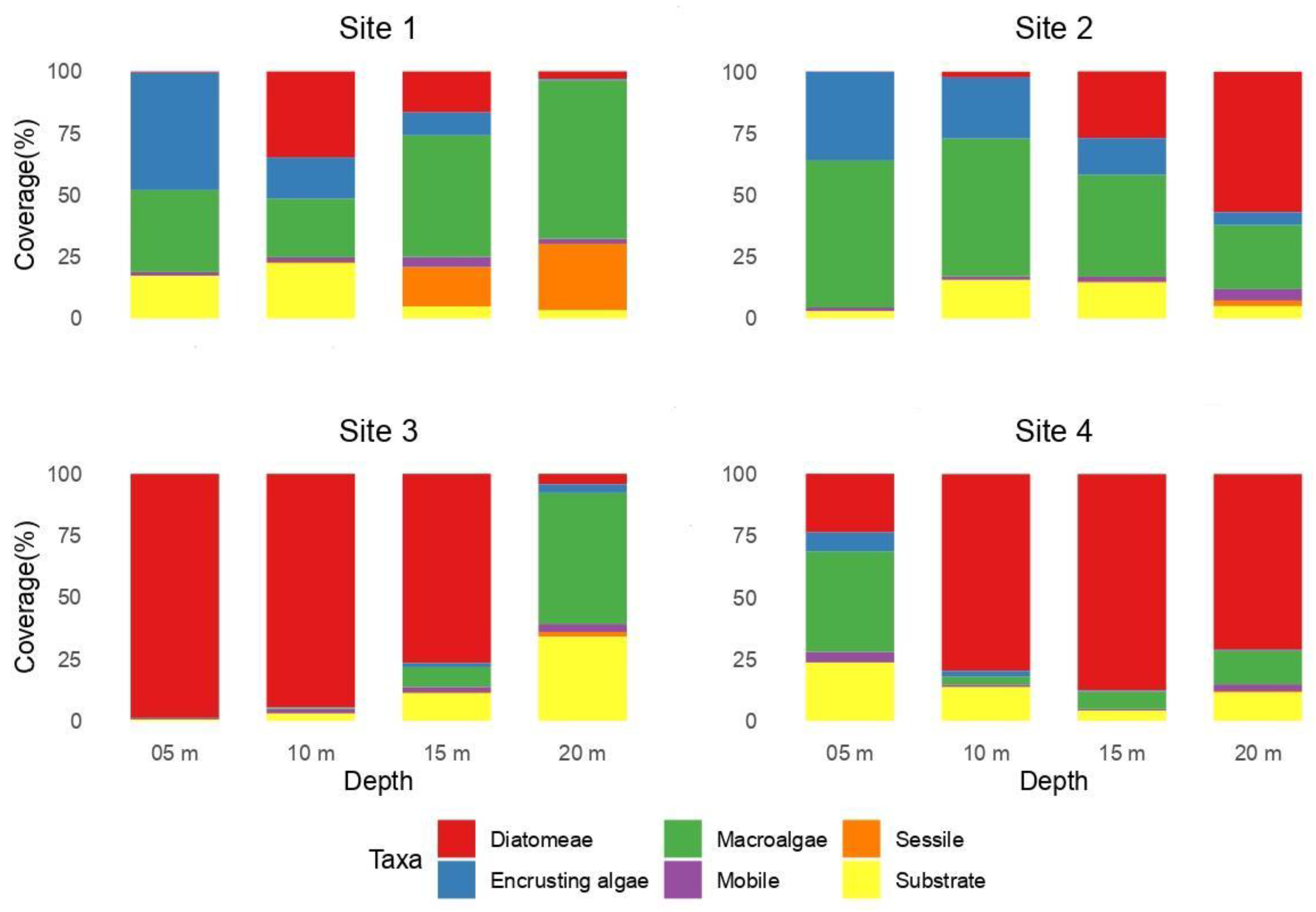
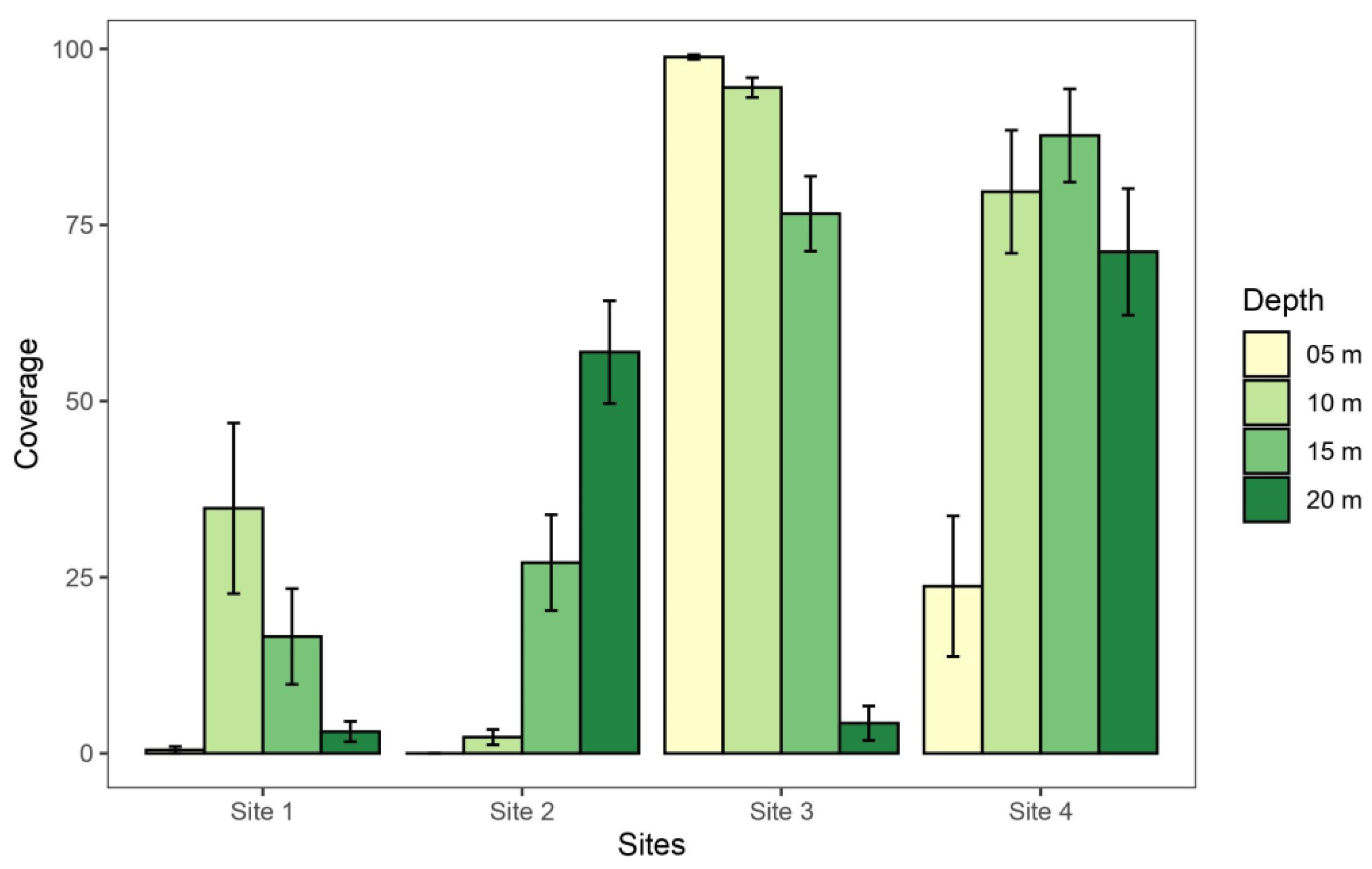
3.3. Multivariate Analysis
4. Discussion
Structural Patterns and Possible Driving Factors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Steig, E.J.; Schneider, D.P.; Rutherford, S.D.; Mann, M.E.; Comiso, J.C.; Shindell, D.T. Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature 2009, 457, 459–462.56. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.A.; González-Aravena, M.; Santibañez, P.A. The importance of local settings: Within-year variability in seawater temperature at South Bay, Western Antarctic Peninsula. PeerJ 2018, 6, e4289. [Google Scholar] [CrossRef] [PubMed]
- Stenni, B.; Curran, M.A.J.; Abram, N.J.; Orsi, A.; Goursaud, S.; Masson-Delmotte, V.; Neukom, R.; Goosse, H.; Divine, D.; van Ommen, T.; et al. Antarctic climate variability on regional and continental scales over the last 2000 years. Clim. Past 2017, 13, 1609–1634. [Google Scholar] [CrossRef]
- Robinson, S.A.; Klekociuk, A.R.; King, D.H.; Pizarro Rojas, M.; Zúñiga, G.E.; Bergstrom, D.M. The 2019/2020 summer of Antarctic heatwaves. Glob. Chang. Biol. 2020, 26, 3178–3180. [Google Scholar] [CrossRef]
- Turner, J.; Marshall, G.J.; Clem, K.; Colwell, S.; Phillips, T.; Lu, H. Antarctic temperature variability and change from station data. Int. J. Climatol. 2020, 40, 2986–3007. [Google Scholar] [CrossRef]
- Turner, J.; Maksym, T.; Phillips, T.; Marshall, G.J.; Meredith, M.P. The impact of changes in sea ice advance on the large winter warming on the western Antarctic Peninsula. Int. J. Climatol. 2013, 33, 852–861. [Google Scholar] [CrossRef]
- Hauri, C.; Doney, S.C.; Takahashi, T.; Erickson, M.; Jiang, G.; Ducklow, H.W. Two decades of inorganic carbon dynamics along the West Antarctic Peninsula. Biogeosciences 2015, 12, 6761–6779. [Google Scholar] [CrossRef]
- Jones, E.M.; Fenton, M.; Meredith, M.P.; Clargo, N.M.; Ossebaar, S.; Ducklow, H.W.; Venables, H.J.; de Baar, H.J. Ocean acidification and calcium carbonate saturation states in the coastal zone of the West Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 139, 181–194. [Google Scholar] [CrossRef]
- Shepherd, A.; Ivins, E.R.; Barletta, V.R.; Bentley, M.J.; Bettadpur, S.; Briggs, K.H.; Bromwich, D.H.; Forsberg, R.; Galin, N.; Horwath, M.; et al. A reconciled estimate of ice-sheet mass balance. Science 2012, 338, 1183–1189. [Google Scholar] [CrossRef]
- Thomas, E.R.; Hosking, J.S.; Tuckwell, R.R.; Warren, R.A.; Ludlow, E.C. Twentieth-century increase in snowfall in coastal West Antarctica. Geophys. Res. Lett. 2015, 42, 9387–9393. [Google Scholar] [CrossRef]
- Thomas, E.R.; Van Wessem, J.M.; Roberts, J.; Isaksson, E.; Schlosser, E.; Fudge, T.J.; Vallelonga, P.; Medley, B.; Lenaerts, J.; Bertler, N.; et al. Regional Antarctic snow accumulation over the past 1000 years. Clim. Past 2017, 13, 1491–1513. [Google Scholar] [CrossRef]
- Morecroft, M.D.; Duffield, S.; Harley, M.; Pearce-Higgins, J.W.; Stevens, N.; Watts, O.; Whitaker, J. Measuring the success of climate change adaptation and mitigation in terrestrial ecosystems. Science 2019, 366, eaaw9256. [Google Scholar] [CrossRef]
- Siegert, M.; Atkinson, A.; Banwell, A.; Brandon, M.; Convey, P.; Davies, B.; Downie, R.; Edwards, T.; Hubbard, B.; Marshall, G.; et al. The Antarctic Peninsula under a 1.5 C global warming scenario. Front. Mar. Sci. 2019, 7, 102. [Google Scholar] [CrossRef]
- Morley, S.A.; Abele, D.; Barnes, D.K.; Cárdenas, C.A.; Cotté, C.; Gutt, J.; Henley, S.F.; Höfer, J.; Hughes, K.A.; Martin, S.M.; et al. Global drivers on Southern Ocean ecosystems: Changing physical environments and anthropogenic pressures in an Earth system. Front. Mar. Sci. 2020, 7, 1097. [Google Scholar] [CrossRef]
- Quartino, M.L.; Deregibus, D.; Campana, G.L.; Latorre, G.E.J.; Momo, F.R. Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in Potter Cove (South Shetland Islands), Antarctica. PLoS ONE 2013, 8, e58223. [Google Scholar] [CrossRef] [PubMed]
- Sahade, R.; Lagger, C.; Torre, L.; Momo, F.; Monien, P.; Schloss, I.; Barnes, D.K.A.; Servetto, N.; Tarantelli, S.; Tatián, M.; et al. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv. 2015, 1, e1500050. [Google Scholar] [CrossRef] [PubMed]
- Lagger, C.; Servetto, N.; Torre, L.; Sahade, R. Benthic colonization in newly ice-free soft bottom areas in an Antarctic fjord. PLoS ONE 2017, 12, e0186756. [Google Scholar] [CrossRef] [PubMed]
- Lagger, C.; Nime, M.; Torre, L.; Servetto, N.; Tatián, M.; Sahade, R. Climate change, glacier retreat and a new ice-free island offer new insights on Antarctic benthic responses. Ecography 2018, 41, 579–591. [Google Scholar] [CrossRef]
- Lee, J.R.; Raymond, B.; Bracegirdle, T.J.; Chadès, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate change drives expansion of Antarctic ice-free habitat. Nature 2017, 547, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, L.; Leclerc, J.C.; Bruning, P.; Garrido, I.; Détrée, C.; Figueroa, A.; Astorga, M.; Navarro, J.M.; Johnson, L.E.; Carlton, J.T.; et al. First mussel settlement observed in Antarctica reveals the potential for future invasions. Sci. Rep. 2020, 10, 5552. [Google Scholar] [CrossRef] [PubMed]
- Abram, N.J.; Mulvaney, R.; Wolff, E.W.; Triest, J.; Kipfstuhl, S.; Trusel, L.D.; Vimeux, F.; Fleet, L.; Arrowsmith, C. Acceleration of snow melt in an Antarctic Peninsula ice core during the twentieth century. Nat. Geosci. 2013, 6, 404–411. [Google Scholar] [CrossRef]
- Paolo, F.S.; Fricker, H.A.; Padman, L. Volume loss from Antarctic ice shelves is accelerating. Science 2015, 348, 327–331. [Google Scholar] [CrossRef]
- Cook, A.J.; Holland, P.R.; Meredith, M.P.; Murray, T.; Luckamn, A.; Vaughan, D.G. Ocean forcing of glacier retreat in the western Antarctic Peninsula. Science 2016, 353, 283–286. [Google Scholar] [CrossRef]
- Turner, J.; Hosking, J.S.; Bracegirdle, T.J.; Marshall, G.J.; Phillips, T. Recent changes in Antarctic sea ice. Philos. Trans. R. Soc. 2015, 373, 20140163. [Google Scholar] [CrossRef] [PubMed]
- Masson, R.A.; Scambos, T.A.; Bennetts, L.G.; Reid, P.; Squire, V.A.; Stammerjohn, S.E. Antarctic ice shelf disintegration triggered by sea ice loss and ocean swell. Nature 2018, 558, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A. Ecological traits of benthic assemblages in shallow Antarctic waters: Does ice scour disturbance select for small, mobile, secondary consumers with high dispersal potential? Polar Biol. 2008, 31, 1225–1231. [Google Scholar] [CrossRef]
- Smale, D.A.; Barnes, D.K.A. Likely responses of the Antarctic benthos to climate-related changes in physical disturbance during the 21st century, based primarily on evidence from the West Antarctic Peninsula region. Ecography 2008, 31, 289–305. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Souster, T. Reduced survival of Antarctic benthos linked to climate-induced iceberg scouring. Nat. Clim. Chang. 2011, 1, 365–368. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Fenton, M.; Cordingley, A. Climate-linked iceberg activity massively reduces spatial competition in Antarctic shallow waters. Curr. Biol. 2014, 24, R553–R554. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A. Iceberg killing fields limit huge potential for benthic blue carbon in Antarctic shallows. Glob. Chang. Biol. 2017, 23, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Gutt, J.; Piepenburg, D. Scale-dependent impact on diversity of Antarctic benthos caused by grounding of icebergs. Mar. Ecol. Prog. Ser. 2003, 253, 77–83. [Google Scholar] [CrossRef]
- Conlan, K.E.; Kvitek, R.G. Recolonization of soft-sediment ice scours on an exposed Arctic coast. Mar. Ecol. Prog. Ser. 2005, 286, 21–42. [Google Scholar] [CrossRef]
- Smale, D.A. Continuous benthic community change along a depth gradient in Antarctic shallows: Evidence of patchiness but not zonation. Polar Biol. 2007, 31, 189–198. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Sublittoral epifaunal communities at Signy Island, Antarctica. I. The ice foot zone. Mar. Biol. 1995, 121, 555–563. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Sublittoral epifaunal communities at Signy Island, Antarctica. II. Below the ice foot zone. Mar. Biol. 1995, 121, 565–572. [Google Scholar] [CrossRef]
- Gutt, J.; Barratt, I.; Domack, E.; d’Acoz, C.D.U.; Dimmler, W.; Grémare, A.; Heilmayer, O.; Isla, E.; Janussen, D.; Jorgensen, E.; et al. Biodiversity change after climate-induced ice-shelf collapse in the Antarctic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 74–83. [Google Scholar] [CrossRef]
- Laudien, J.; Orchard, J.B. The significance of depth and substratum incline for the structure of a hard bottom sublittoral community in glacial Kongsfjorden (Svalbard, Arctic)—An underwater imagery approach. Polar Biol. 2012, 35, 1057–1072. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; Newcombe, E.M.; Hajdu, E.; Gonzalez-Aravena, M.; Geange, S.W.; Bell, J.J. Sponge richness on algae-dominated rocky reefs in the Western Antarctic Peninsula and the Magellan Strait. Polar Res. 2016, 35, 30532. [Google Scholar] [CrossRef]
- Bravo, D. Análisis de Patrones de Biodiversidad, Distribución Espacial y Abundancia del Ensamble de Esponjas en Isla Doumer (Archipiélago de Palmer: Península Antártica) Licenciado en Biología Marina. Ph.D. Thesis, Universidad de Valparaíso, Valparaíso, Chile, 2017. [Google Scholar]
- Fernandez, J.C.; Bravo-Gomez, D.; Cárdenas, C.A.; Hajdu, E. Sponges from Doumer Island, Antarctic Peninsula, with description of new species of Clathria (Axosuberites) Topsent, 1893 and Hymeniacidon Bowerbank, 1858, and a re-description of H. torquata Topsent, 1916. Zootaxa 2020, 4728, 77–109. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.A.; Font, A.; Steinert, G.; Rondon, R.; González-Aravena, M. Temporal stability of bacterial communities in Antarctic sponges. Front. Microbiol. 2019, 10, 2699. [Google Scholar] [CrossRef]
- Happel, L.; Rondon, R.; Font, A.; González-Aravena, M.; Cárdenas, C.A. Stability of the microbiome of the sponge Mycale (Oxymycale) acerata in the Western Antarctic Peninsula. Front. Microbiol. 2022, 759, 827863. [Google Scholar] [CrossRef]
- González-Aravena, M.; Kenny, N.J.; Osorio, M.; Font, A.; Riesgo, A.; Cárdenas, C.A. Warm temperatures, cool sponges: The effect of increased temperatures on the Antarctic sponge Isodictya sp. PeerJ 2019, 7, e8088. [Google Scholar] [CrossRef]
- Rovelli, L.; Attard, K.M.; Cárdenas, C.A.; Glud, R.N. Benthic primary production and respiration of shallow rocky habitats: A case study from South Bay (Doumer Island, Western Antarctic Peninsula). Polar Biol. 2019, 42, 1459–1474. [Google Scholar] [CrossRef]
- Zamorano, J.H. Zonación y Biomasa de la Macrofauna Bentónica en Bahía South, Archipiélago de Palmer, Antártica. INACH Ser. Científica 1983, 30, 27–38. [Google Scholar]
- Zamorano, J.H.; Duarte, W.E.; Moreno, C.A. Predation upon Laternula elliptica (Bivalvia, Anatinidae): A field manipulation in South Bay, Antarctica. Polar Biol. 1986, 6, 139–143. [Google Scholar] [CrossRef]
- Villegas, N.; Málikov, I.; Cárdenas, C. An initial aproximation to the meteo-marine conditions of South Bay (Doumer island) and comparison of the meteorological behaviour between Doumer and Anvers islands, Antarctica (austral summer 2016–2017). An. Del Inst. De La Patagon. 2018, 46, 23–32. [Google Scholar] [CrossRef]
- Sentinel Hub. EO Browser. Sentinel Hub. 2020. Available online: https://apps.sentinel-hub.com/eo-browser/ (accessed on 13 March 2020).
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2013; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2013.
- Clarke, K.R.; Warwick, R.M. Change in marine communities. Approach Stat. Anal. Interpret. 2001, 2, 1–168. [Google Scholar]
- Cavanagh, R.D.; Melbourne-Thomas, J.; Grant, S.M.; Barnes, D.K.A.; Hughes, K.A.; Halfter, S.; Meredith, M.P.; Murphy, E.J.; Trebilco, R.; Hill, S.L. Future risk for Southern Ocean ecosystem services under climate change. Front. Mar. Sci. 2021, 7, 615214. [Google Scholar] [CrossRef]
- Sahade, R.; Tatián, M.; Kowalke, J.; Kühne, S.; Esnal, G.B. Benthic faunal associations on soft substrates at Potter Cove, King George Island, Antarctica. Polar Biol. 1998, 19, 85–91. [Google Scholar] [CrossRef]
- Lovell, L.L.; Trego, K.D. The epibenthic megafaunal and benthic infaunal invertebrates of Port Foster, Deception Island (South Shetland Islands, Antarctica). Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 1799–1819. [Google Scholar] [CrossRef]
- Gutt, J. Antarctic macro-zoobenthic communities: A review and an ecological classification. Antarct. Sci. 2007, 19, 165–182. [Google Scholar] [CrossRef]
- Angulo-Preckler, C.; Figuerola, B.; Núñez-Pons, L.; Moles, J.; Martín-Martín, R.; Rull-Lluch, J.; Gómez-Garreta, A.; Avila, C. Macrobenthic patterns at the shallow marine waters in the caldera of the active volcano of Deception Island, Antarctica. Cont. Shelf Res. 2018, 157, 20–31. [Google Scholar] [CrossRef]
- Bowden, D.A. Seasonality of recruitment in Antarctic sessile marine benthos. Mar. Ecol. Prog. Ser. 2005, 297, 101–118. [Google Scholar] [CrossRef]
- White, B.A.; McClintock, J.; Amsler, C.D.; Mah, C.L.; Amsler, M.O.; White, S.; Quetin, L.B.; Ross, R.M. The abundance and distribution of echinoderms in nearshore hard-bottom habitats near Anvers Island, western Antarctic Peninsula. Antarct. Sci. 2012, 24, 554. [Google Scholar] [CrossRef]
- Amsler, C.D.; Amsler, M.O.; Klein, A.G.; Galloway, A.W.; Iken, K.; McClintock, J.B.; Heiser, S.; Lowe, A.T.; Schram, J.B.; Whippo, R. Strong correlations of sea ice cover with macroalgal cover along the Antarctic Peninsula: Ramifications for present and future benthic communities. Elementa Sci. Anthrop. 2023, 11, 00020. [Google Scholar] [CrossRef]
- Ericson, J.A.; Ho, M.A.; Miskelly, A.; King, C.K.; Virtue, P.; Tilbrook, B.; Byrne, M. Combined effects of two ocean change stressors, warming and acidification, on fertilization and early development of the Antarctic echinoid Sterechinus neumayeri. Polar Biol. 2012, 35, 1027–1034. [Google Scholar] [CrossRef]
- Suckling, C.C.; Clark, M.S.; Richard, J.; Morley, S.A.; Thorne, M.A.; Harper, E.M.; Peck, L.S. Adult acclimation to combined temperature and p H stressors significantly enhances reproductive outcomes compared to short-term exposures. J. Anim. Ecol. 2015, 84, 773–784. [Google Scholar] [CrossRef]
- Dayton, P.K.; Jarrell, S.C.; Kim, S.; Parnell, P.E.; Thrush, S.F.; Hammerstrom, K.; Leichter, J.J. Benthic responses to an Antarctic regime shift: Food particle size and recruitment biology. Ecol. Appl. 2019, 29, e01823. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.Y.; Moon, H.W.; Jeon, M.; Kang, S.H. First record of massive blooming of benthic diatoms and their association with megabenthic filter feeders on the shallow seafloor of an Antarctic Fjord: Does glacier melting fuel the bloom? Ocean Sci. J. 2016, 51, 273–279. [Google Scholar] [CrossRef]
- Pasotti, F.; Manini, E.; Giovannelli, D.; Wölfl, A.C.; Monien, D.; Verleyen, E.; Braeckman, U.; Abele, D.; Vanreusel, A. Antarctic shallow water benthos in an area of recent rapid glacier retreat. Mar. Ecol. 2015, 36, 716–733. [Google Scholar] [CrossRef]
- Campana, G.L.; Zacher, K.; Deregibus, D.; Momo, F.R.; Wiencke, C.; Quartino, M.L. Succession of Antarctic benthic algae (Potter Cove, South Shetland Islands): Structural patterns and glacial impact over a four-year period. Polar Biol. 2017, 41, 377–396. [Google Scholar] [CrossRef]
- Ha, S.Y.; Ahn, I.Y.; Moon, H.W.; Choi, B.; Shin, K.H. Tight trophic association between benthic diatom blooms and shallow-water megabenthic communities in a rapidly deglaciated Antarctic fjord. Estuar. Coast. Shelf Sci. 2019, 218, 258–267. [Google Scholar] [CrossRef]
- Zidarova, R.; Ivanov, P.; Dzhembekova, N. Diatom colonization and community development in Antarctic marine waters–a short-term experiment. Pol. Polar Res. 2020, 41, 187–212. [Google Scholar] [CrossRef]
- Bae, H.; Ahn, I.Y.; Park, J.; Song, S.J.; Noh, J.; Kim, H.; Khim, J.S. Shift in polar benthic community structure in a fast retreating glacial area of Marian Cove, West Antarctica. Sci. Rep. 2021, 11, 241. [Google Scholar] [CrossRef]
- Wright, J.T.; Benkendorff, K.; Davis, A.R. Habitat associated differences in temperate sponge assemblages: The importance of chemical defence. J. Exp. Mar. Biol. Ecol. 1997, 213, 199–213. [Google Scholar] [CrossRef]
- Ávila, E.; Blancas-Gallangos, N.I.; Riosmena-Rodríguez, R.; Paul-Chávez, L. Sponges associated with Sargassum spp. (Phaeophyceae: Fucales) from the south-western Gulf of California. J. Mar. Biolog. Assoc. UK 2010, 90, 193–202. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; Davy, S.K.; Bell, J.J. Influence of canopy-forming algae on temperate sponge assemblages. J. Mar. Biolog. Assoc. UK 2016, 96, 351–362. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- Zenteno-Devaud, L.; Aguirre-Martinez, G.V.; Andrade, C.; Cárdenas, L.; Pardo, L.M.; González, H.E.; Garrido, I. Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula. Biology 2022, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Brockington, S. Zoobenthic biodiversity, biomass and abundance at Adelaide Island, Antarctica. Mar. Ecol. Prog. Ser. 2003, 249, 145–155. [Google Scholar] [CrossRef]
- Nonato, E.F.; Brito, T.A.; De Paiva, P.C.; Petti, M.A.; Corbisier, T.N. Benthic megafauna of the nearshore zone of Martel Inlet (King George Island, South Shetland Islands, Antarctica): Depth zonation and underwater observations. Polar Biol. 2000, 23, 580–588. [Google Scholar] [CrossRef]
- Jørgensen, L.L.; Gulliksen, B. Rocky bottom fauna in arctic Kongsfjord (Svalbard) studied by means of suction sampling and photography. Polar Biol. 2001, 24, 113–121. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; Montiel, A. The influence of depth and substrate inclination on sessile assemblages in subantarctic rocky reefs (Magellan region). Polar Biol. 2015, 38, 1631–1644. [Google Scholar] [CrossRef]
- Leichter, J.J.; Witman, J.D. Water flow over subtidal rock walls: Relation to distributions and growth rates of sessile suspension feeders in the Gulf of Maine Water flow and growth rates. J. Exp. Mar. Biol. Ecol. 1997, 209, 293–307. [Google Scholar] [CrossRef]
- Clark, G.F.; Stark, J.S.; Palmer, A.S.; Riddle, M.J.; Johnston, E.L. The roles of sea-ice, light and sedimentation in structuring shallow Antarctic benthic communities. PLoS ONE 2017, 12, e0168391. [Google Scholar] [CrossRef]


| Site 1 | Site 2 | Site 3 | Site 4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Class | Taxon | 5 m | 10 m | 15 m | 20 m | 5 m | 10 m | 15 m | 20 m | 5 m | 10 m | 15 m | 20 m | 5 m | 10 m | 15 m | 20 m |
| Porifera | Demospongiae | Mycale (Oxymycale) acerata | ● | ● | ● ● | ● | ||||||||||||
| Mycale sp. | ● | ● | ● | ● | ||||||||||||||
| Sphaerotylus antarcticus | ●● | ● | ● | |||||||||||||||
| Dendrilla antarctica | ● | ● | ● | ● | ||||||||||||||
| Demospongiae sp. 1 | ● | ● | ||||||||||||||||
| Demospongiae sp. 2 | ● | |||||||||||||||||
| Haliclona sp. | ● | ● | ||||||||||||||||
| Haliclona (Soestella) sp. | ● | ● | ||||||||||||||||
| Haliclona (Rhizoniera) sp. | ● | |||||||||||||||||
| Clathria (Axosuberites) sp. | ● | |||||||||||||||||
| Tedania (Tedaniopsis) sp. | ● | |||||||||||||||||
| Hymeniacidon sp. | ● | |||||||||||||||||
| Porifera indet. 1 | ● | |||||||||||||||||
| Porifera indet. 2 | ● | |||||||||||||||||
| Porifera indet. 3 | ● | ● | ||||||||||||||||
| Cnidaria | Anthozoa | Clavularia sp. | ●● | ●●●●● | ●● | ● | ● | ● | ●● | ● | ||||||||
| Urticinopsis antarctica | ● | ● | ||||||||||||||||
| Hormosoma scotti | ● | |||||||||||||||||
| Anthozoa indet. 1 | ● | |||||||||||||||||
| Bryozoa | Bryozoan indet. 1 | ● | ||||||||||||||||
| Brachiopoda | Brachiopod indet. 1 | ● | ● | |||||||||||||||
| Nemertea | Anopla | Parbolasia corrugatus | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||
| Mollusca | Gastropoda | Doris kerguelenensis | ● | ● | ||||||||||||||
| Margarella antarctica | ● | ● | ● | ● | ● | |||||||||||||
| Nacella concinna | ●● | ●● | ● | ● | ●● | ●● | ●●●● | ●● | ● | ● | ||||||||
| Gastropod indet. 1 | ● | |||||||||||||||||
| Echinodermata | Crinoidea | Promachocrinus kerguelensis | ● | |||||||||||||||
| Asteroidea | Odontaster validus | ● | ● | ● | ●● | ●● | ●● | ● | ● | ●● | ●●● | ●● | ● | ●● | ● | ● | ||
| Odontaster meridionalis | ● | ● | ● | ● | ||||||||||||||
| Granaster nutrix | ●● | ● | ● | ●● | ●● | ● | ●● | ● | ● | ● | ● | ● | ● | |||||
| Labidiaster annulatus | ● | ● | ● | ● | ||||||||||||||
| Perknaster sp. | ● | ● | ||||||||||||||||
| Asteroidea indet. 1 | ● | ● | ● | ● | ● | ● | ||||||||||||
| Asteroidea indet. 2 | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||
| Holoturoidea | Heterocucumis steineni | ● | ●● | ● | ●● | ●● | ● | ● | ●● | |||||||||
| Chordata | Ascidiacea | Cnemidocarpa verrucosa | ● | ● | ● | |||||||||||||
| Corella antarctica | ● | ● | ||||||||||||||||
| Pyura setosa | ● | ● | ● | |||||||||||||||
| Protoctista | Benthic filamentous diatoms | |||||||||||||||||
| Chlorophyta | Ulvophyceae | Green algae indet. 1 | ||||||||||||||||
| Green algae indet. 2 | ||||||||||||||||||
| Green algae indet. 3 | ||||||||||||||||||
| Monostroma hariotii | ||||||||||||||||||
| Rhodophyta | Florideophyceae | Paraglossum sp. | ||||||||||||||||
| Sarcopeltis antarcticus | ||||||||||||||||||
| Palmaria decipiens | ||||||||||||||||||
| Plocamium cartilagineum | ||||||||||||||||||
| Trematocarpus antarcticus | ||||||||||||||||||
| Pink encrusting algae | ||||||||||||||||||
| Red encrusting algae | ||||||||||||||||||
| White encrusting algae | ||||||||||||||||||
| Yellow encrusting algae | ||||||||||||||||||
| Encrusting algae indet. | ||||||||||||||||||
| Pink coralline algae indet. | ||||||||||||||||||
| Red algae indet. 1 | ||||||||||||||||||
| Red algae indet. 2 | ||||||||||||||||||
| Red algae indet. 3Morphospecie indet. | ||||||||||||||||||
| Ochrophyta | Phaeophyceae | Desmarestia sp. | ||||||||||||||||
| Himantothallus grandifolius | ||||||||||||||||||
| Diversity | Sites | Depth | |||||||
| S1 | S2 | S3 | S4 | 5 m | 10 m | 15 m | 20 m | ||
| S’ | 52 | 34 | 27 | 31 | 21 | 28 | 54 | 53 | |
| H’ | 1.35 b | 1.23 b | 0.58 a | 0.47 a | 0.81 bc | 0.70 c | 0.89 b | 1.24 a | |
| Sites | Depth | |||||||
| S3 | S1 | S2 | 5 m | 10 m | 15 m | |||
| S1 | 0.35 * | 10 m | 0.15 * | |||||
| S2 | 0.39 * | 0.06 * | 15 m | 0.25 * | 0.04 * | |||
| S4 | 0.06 * | 0.32 * | 0.33 * | 20 m | 0.28 * | 0.14 * | 0.08 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, S.; Cárdenas, C.A.; Bravo-Gómez, D.; Lagger, C. Shallow Hard-Bottom Benthic Assemblages of South Bay (Antarctic Peninsula): An Update 40 Years Later. Diversity 2024, 16, 162. https://doi.org/10.3390/d16030162
Morales S, Cárdenas CA, Bravo-Gómez D, Lagger C. Shallow Hard-Bottom Benthic Assemblages of South Bay (Antarctic Peninsula): An Update 40 Years Later. Diversity. 2024; 16(3):162. https://doi.org/10.3390/d16030162
Chicago/Turabian StyleMorales, Sol, César A. Cárdenas, Diego Bravo-Gómez, and Cristian Lagger. 2024. "Shallow Hard-Bottom Benthic Assemblages of South Bay (Antarctic Peninsula): An Update 40 Years Later" Diversity 16, no. 3: 162. https://doi.org/10.3390/d16030162






