From Points to Forecasts: Predicting Invasive Species Habitat Suitability in the Near Term
Abstract
:1. Introduction
1.1. Methods
Data
| Scientific name | Common name | Sample size | Training AUC | Test AUC | Threshold value | Area on Leading Edge of invasion (~km2) | Area on Trailing Edge of invasion (~km2) | Change in Area(~km2) | Habitat specialist? | Introduced after 1900? |
|---|---|---|---|---|---|---|---|---|---|---|
| Bromus tectorum | Cheat grass | 9517 | 0.84 | 0.83 | 0.43 | 131,000 | 131,000 | 0 | No | No |
| Carduus nutans | Muskthistle | 4670 | 0.88 | 0.88 | 0.39 | 372,000 | 383,000 | −11,000 | No | No |
| Celastrus orbiculatus | Oriental bittersweet | 282 | 0.84 | 0.79 | 0.24 | 103,000 | 156,000 | −53,000 | No | No |
| Centaurea stoebe | Spotted knapweed | 5899 | 0.91 | 0.90 | 0.46 | 195,000 | 245,000 | −50,000 | No | No |
| Cirsium arvense | Canada thistle | 7960 | 0.86 | 0.86 | 0.45 | 206,000 | 423,000 | −217,000 | No | No |
| Cynoglossum officinale | Houndstounge | 1884 | 0.89 | 0.88 | 0.34 | 228,000 | 219,000 | 9,000 | No | No |
| Lepidium latifolium | Perrennial pepperweed | 1015 | 0.93 | 0.91 | 0.36 | 546,000 | 451,000 | 95,000 | No | Yes |
| Linaria dalmatica | Dalmation toadflax | 1372 | 0.92 | 0.91 | 0.39 | 239,000 | 284,000 | −45,000 | No | No |
| Lonicera japonica | Japaneese honeysuckle | 2771 | 0.70 | 0.68 | 0.40 | 170,000 | 144,000 | 26,000 | No | No |
| Lythrum salicaria | Purple loosestrife | 4921 | 0.85 | 0.84 | 0.37 | 376,000 | 293,000 | 83,000 | Yes | No |
| Microstegium vimineum | Japanese stiltgrass | 321 | 0.78 | 0.68 | 0.34 | 243,000 | 110,000 | 133,000 | No | Yes |
| Pennisetum ciliare | Buffelgrass | 1876 | 0.92 | 0.91 | 0.37 | 20,000 | 20,000 | 0 | Yes | Yes |
Modeling Techniques
2. Results and Discussion
2.1. Climate Scenarios
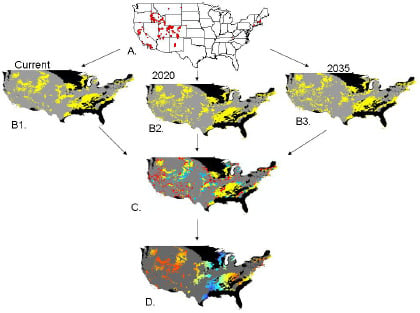
2.2. Invasibility Index

2.3. Utility of This Approach
3. Experimental Section


| Variable | Percent Contribution |
|---|---|
| Mean Diurnal Range | 24 |
| Precipitation of Warmest Quarter | 16 |
| Min Temperature of Coldest Month | 12 |
| Mean Temperature of Driest Quarter | 10 |
| Mean Temperature of Wettest Quarter | 10 |
| Mean Temperature of Warmest Quarter | 8 |
| Precipitation of Wettest Month | 7 |
| Precipitation Seasonality | 4 |
| Precipitation of Driest Month | 4 |
| Isothermality | 4 |
3.1. Invasibility Index Map
4. Conclusions
Acknowledgements
References
- Anderson, L.W.J. Control of invasive seaweeds. Bot. Mar. 2007, 50, 418–437. [Google Scholar]
- Noonburg, E.G.; Byers, J.E. More harm than good: When invader vulnerability to predators enhances impact on native species. Ecology 2005, 86, 2555–2560. [Google Scholar] [CrossRef]
- Snyder, W.E.; Evans, E.W. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. S. 2006, 37, 95–122. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. Bioscience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Leung, B.; Lodge, D.M.; Finnoff, D.; Shogren, J.F.; Lewis, M.A.; Lamberti, G. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc. R. Soc. B 2002, 269, 2407–2413. [Google Scholar] [CrossRef]
- Moody, M.E.; Mack, R.N. Controlling the spread of plant invasions: The importance of nascent foci. J. App. Ecol. 1988, 25, 1009–1021. [Google Scholar] [CrossRef]
- Rejmanek, M.; Pitcairn, M.J. When is eradication of exotic pest plants a realistic goal? In Turning the Tide: The Eradication of Invasive Species; Veitch, C.R., Clout, M.N., Eds.; IUCN SSC Invasive Species Specialist Group: IUCN, Gland, Switzerland and Cambridge, UK, 2002; pp. 249–253. [Google Scholar]
- Thuiller, W.; Albert, C.; Araujo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgely, G.F.; Paterson, J.; Schurr, F.M.; Sykes, M.T.; Zimmermann, N.E. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Dantonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Bradley, B.A.; Oppenheimer, M.; Wilcove, D.S. Climate Change and plant invasions: restoration opportunities ahead? Global Change Biol. 2009. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Stohlgren, T.J.; Barnett, D.T.; Jarnevich, C.S.; Flather, C.; Kartesz, J. The myth of plant species saturation. Ecol. Lett. 2008, 11, 313–322. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Graham, C.H. The ability of climate envelope models to predict the effect of climate change on species distributions. Global Change Biol. 2006, 12, 2272–2281. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Stohlgren, T.J. Measuring Plant Diversity: Lessons from the Field; Oxford University Press: Oxford, UK; New York, NY, USA, 2007. [Google Scholar]
- Stohlgren, T.J.; Schnase, J.L. Risk analysis for biological hazards: What we need to know about invasive species. Risk Anal. 2006, 26, 163–173. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Morisette, J.T.; Jarnevich, C.S.; Ullah, A.; Cai, W.J.; Pedelty, J.A.; Gentle, J.E.; Stohlgren, T.J.; Schnase, J L. A tamarisk habitat suitability map for the continental United States. Front. Ecol. Environ. 2006, 4, 11–17. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Thuiller, W.; Miaud, C. Prediction and validation of the potential global distribution of a problematic alien invasive species—the American bullfrog. Divers. Distrib. 2007, 13, 476–485. [Google Scholar] [CrossRef]
- Galatowitsch, S.M.; Anderson, N.O.; Ascher, P.D. Invasiveness in wetland plants in temperate North America. Wetlands 1999, 19, 733–755. [Google Scholar] [CrossRef]
- Ibarraf, F.A.; Cox, J.R.; Martinr, M.H.; Crowl, T.A.; Call, C.A. Predicting buffelgrass survival across a geographical and environmental gradient. J. Range Manag. 1995, 48, 53–59. [Google Scholar] [CrossRef]
- NIISS National Institute of Invasive Species Science. Available online: http://www.niiss.org/ (accessed February 29, 2010).
- Graham, J.; Newman, G.; Jarnevich, C.; Shory, R.; Stohlgren, T.J. A global organism detection and monitoring system for non-native species. Ecol. Inform. 2007, 2, 177–183. [Google Scholar]
- Nix, H.A. A biogeographic analysis of Australian elapid snakes. In Atlas of Elapid Snakes of Australia; Longmore, R., Ed.; Australian Government Publishing Service: Canberra, Australia, 1986. [Google Scholar]
- DAYMET. Climatological summaries for the conterminous United States, 1980–1997. Daily Surface Weather and Climatological Summaries. 2006. Available online: http://daymet.org (accessed March 31, 2009).
- Daly, C.; Gibson, W.P.; Taylor, G.H.; Johnson, G.L.; Pasteris, P. A knowledge-based approach to the statistical mapping of climate. Clim. Res. 2002, 22, 99–113. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Stohlgren, T.J. Near term climate projections for invasive species distributions. Biol. Invasions 2009, 11, 1373–1379. [Google Scholar] [CrossRef]
- SSI. SYSTAT 12.0; Systat Software, Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Phillips, S.J.; Dudik, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef]
- ESRI. ArcGIS 9.3; Environmental Systems Research Institute, Inc.: Redlands, CA, USA, 2008. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar]
- Scott, J.M.; Heglund, P.; Morrison, M.L.; Raven, P.H. Predicting Species Occurrences: Issues of Accuracy and Scale; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Lobo, J.M.; Jimenez-Valverde, A.; Real, R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Hodkinson, D.J.; Thompson, K. Plant dispersal: the role of man. J. App. Ecol. 1997, 34, 1484–1496. [Google Scholar] [CrossRef]
- Mack, R.N.; Lonsdale, W.M. Humans as global plant dispersers: Getting more than we bargained for. Bioscience 2001, 51, 95–102. [Google Scholar] [CrossRef]
- Reichard, S.H.; White, P. Horticulture as a pathway of invasive plant introductions in the United States. Bioscience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar]
- Beaumont, L.J.; Pitman, A.J.; Poulsen, M.; Hughes, L. Where will species go? Incorporating new advances in climate modelling into projections of species distributions. Global Change Biol. 2007, 13, 1368–1385. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; Li, J.; Lohmann, L.G.; Loiselle, B.A.; Manion, G.; Moritz, C.; Nakamura, M.; Nakazawa, Y.; Overton, J.M.; Peterson, A.T.; Phillips, S.J.; Richardson, K.; Scachetti-Pereira, R.; Schapire, R.E.; Soberon, J.; Williams, S.; Wisz, M.S.; Zimmermann, N.E. Novel methods improve prediction of species' distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Evangelista, P.H.; Kumar, S.; Stohlgren, T.J.; Jarnevich, C.S.; Crall, A.W.; Norman, J.B.; Barnett, D.T. Modelling invasion for a habitat generalist and a specialist plant species. Divers. Distrib. 2008, 14, 808–817. [Google Scholar] [CrossRef]
- Leathwick, J.R.; Austin, M.P. Competitive interactions between tree species in New Zealand's old-growth indigenous forests. Ecology 2001, 82, 2560–2573. [Google Scholar] [CrossRef]
- Anderson, R.P.; Peterson, A.T.; Gomez-Laverde, M. Using niche-based GIS modeling to test geographic predictions of competitive exclusion and competitive release in South American pocket mice. Oikos 2002, 98, 3–16. [Google Scholar] [CrossRef]
- Anderson, B.J.; Akcakaya, H.R.; Araujo, M.B.; Fordham, D.A.; Martinez-Meyer, E.; Thuiller, W.; Brook, B.W. Dynamics of range margins for metapopulations under climate change. Proc. R. Soc. B 2009, 276, 1415–1420. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. JENE. 2009, 1, 94–98. [Google Scholar]
- Kumar, S.; Spaulding, S.A.; Stohlgren, T.J.; Hermann, K.A.; Schmidt, T.S.; Bahls, L.L. Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front. Ecol. Environ. 2009, 7, 415–420. [Google Scholar]
- Araujo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol.Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Stohigren, T.J.; Ma, P.; Kumar, S.; Rocca, M.; Morisette, J.T.; Jarnevich, C.S.; Benson, N. Ensemble habitat mapping of invasive plant species. Risk Anal. 2009, 30, 224–235. [Google Scholar]
- Crosier, C.S. Synergistic Methods to Generate Predictive Models at Large Spatial Extents and Fine Resolution; Colorado State University: Fort Collins, CO, USA, 2004. [Google Scholar]
- Stohlgren, T.J.; Jarnevich, C.; Chong, G.W.; Evangelista, P.H. Scale and plant invasions: a theory of biotic acceptance. Preslia 2006, 78, 405–426. [Google Scholar]
| Data source | Citation |
|---|---|
| Crosier PhD - Department of Transportation | [52] |
| Crosier PhD - Larimer County | [52] |
| Crosier PhD - San Luis Valley | [52] |
| Crosier PhD - The Nature Conservancy | [52] |
| Crosier PhD - Jackson County | [52] |
| Crosier PhD - Larimer County | [52] |
| Crosier PhD - Otero | [52] |
| Crosier PhD - Royal Gorge | [52] |
| Crosier PhD - San Luis Valley | [52] |
| Crosier PhD - Colorado State Parks | [52] |
| Crosier PhD - CNHP | [52] |
| Florida Natural Areas Inventory | http://fnai.org/invasivespecies.cfm |
| Florida Natural Areas Inventory | http://fnai.org/invasivespecies.cfm |
| The Great Lakes Indian Fish & Wildlife Commission | http://www.glifwc.org/ |
| Idaho State Department of Agriculture | Invasive Species Coordinator |
| Invasive Plant Atlas of the MidSouth | http://www.gri.msstate.edu/ipams/ |
| Invasive Plant Atlas of New England | http://nbii-nin.ciesin.columbia.edu/ipane/ |
| Montana Fish, Wildlife, and Parks | http://fwp.mt.gov/insidefwp/gis/shapefiles/fasweeds.zip |
| Modified Whittaker Plot Information | [53] |
| National Institute of Invasive Species Science project - Air Force Academy Weed Mapping | www.NIISS.org |
| National Institute of Invasive Species Science project - Bohemian Foundation | www.NIISS.org |
| National Institute of Invasive Species Science project - Colorado | www.NIISS.org |
| National Institute of Invasive Species Science project - ELK | www.NIISS.org |
| National Institute of Invasive Species Science project - Grand Staircase Escalante National Monument | www.NIISS.org |
| National Institute of Invasive Species Science project - Grazing effects | www.NIISS.org |
| National Institute of Invasive Species Science project - GVM Weed Test | www.NIISS.org |
| National Institute of Invasive Species Science project - Hart Mountain National Antelope | www.NIISS.org |
| National Institute of Invasive Species Science project - Highway 24 Weed Mapping | www.NIISS.org |
| National Institute of Invasive Species Science project - Invasive Carduus Thistles | www.NIISS.org |
| National Institute of Invasive Species Science project - National Elk Refuge | www.NIISS.org |
| National Institute of Invasive Species Science project - National Wildlife Refuge - USGS | www.NIISS.org |
| National Institute of Invasive Species Science project - Nevada Cheatgrass | www.NIISS.org |
| National Institute of Invasive Species Science project - New Invaders Watch List | www.NIISS.org |
| National Institute of Invasive Species Science project - Peterson Air Force Base Weed Mapping | www.NIISS.org |
| National Institute of Invasive Species Science project - Plains Riparian study | www.NIISS.org |
| National Institute of Invasive Species Science project - Plants of Concern | www.NIISS.org |
| National Institute of Invasive Species Science project - Pondicherry National Wildlife | www.NIISS.org |
| National Institute of Invasive Species Science project - Rocky Mountain NP LANDGAP | www.NIISS.org |
| National Institute of Invasive Species Science project - SAIN Invasive Plants | www.NIISS.org |
| National Institute of Invasive Species Science project - SE-EPPC EDDMaps | www.NIISS.org |
| National Institute of Invasive Species Science project - September 2007 Training at the ELC | www.NIISS.org |
| National Institute of Invasive Species Science project - Wisconsin Invasive Plants of the Future | www.NIISS.org |
| National Institute of Invasive Species Science project - Colorado Department of Transportation | www.NIISS.org |
| National Institute of Invasive Species Science project - Hart Mountain National Antelope | www.NIISS.org |
| National Institute of Invasive Species Science project - National Bison Range | www.NIISS.org |
| Personal Collection of Robert K. Peet | The University of North Carolina at Chapel Hill |
| Personal Collection of James F. Quinn | University of California, Davis |
| Southwest Exotic Mapping Program | http://sbsc.wr.usgs.gov/research/projects/swepic/swemp/swempA.asp |
| Bureau of Land Management, Utah State Office | Salt Lake City, UT |
| TexasInvasives.org | http://www.texasinvasives.org/ |












© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holcombe, T.R.; Stohlgren, T.J.; Jarnevich, C.S. From Points to Forecasts: Predicting Invasive Species Habitat Suitability in the Near Term. Diversity 2010, 2, 738-767. https://doi.org/10.3390/d2050738
Holcombe TR, Stohlgren TJ, Jarnevich CS. From Points to Forecasts: Predicting Invasive Species Habitat Suitability in the Near Term. Diversity. 2010; 2(5):738-767. https://doi.org/10.3390/d2050738
Chicago/Turabian StyleHolcombe, Tracy R., Thomas J. Stohlgren, and Catherine S. Jarnevich. 2010. "From Points to Forecasts: Predicting Invasive Species Habitat Suitability in the Near Term" Diversity 2, no. 5: 738-767. https://doi.org/10.3390/d2050738