Predicting Climate Change Impacts to the Canadian Boreal Forest
Abstract
:1. Introduction
- (1).
- Modelled possible spatial distributions of vegetation productivity across Canada’s boreal in 2020, 2050, and 2080, using historical climate data, linked to historical remote sensing derived indirect indicators of vegetation productivity from 1987–2007, which are then projected using a range of future climate scenarios;
- (2).
- Assessed the performance of predictive models by comparing observed and predicted maps of vegetation productivity using a fuzzy map comparison approach, and
- (3).
- Mapped temporal variability in productivity predictions (from 2020, 2050, and 2080) to determine the locations where vegetation appears most and least sensitive to climate change.
2. Methods
2.1. Study Area and Data

2.1.1. Ecodistricts and Ecozones
2.1.2. Indirect Biodiversity Indicators
| Ecozone | Landforms | Climate/ oceanographic characteristics | Vegetation/ productivity | Area (km2) |
|---|---|---|---|---|
| Arctic Cordillera | Mountains | Extremely cold, dry; continuous permafrost | Mainly unvegetated; some shrub-herb tundra | 230,873 |
| Atlantic Maritime | Hills and coastal plains | Cool, wet | Mixed deciduous-evergreen forest | 183,978 |
| Boreal Cordillera | Mountains, some hills | Moderately cold, moist | Largely evergreen forest; some tundra, open woodland | 459,680 |
| Boreal Plains | Plains, some foothills | Cold, moist | Mixed evergreen-deciduous forest | 679,969 |
| Boreal Shield East | Plains, some hills | Cold, moist | Evergreen forest, mixed evergreen, deciduous forest | 1,782,252 |
| Boreal Shield West | ||||
| Hudson Plains | Plains | Cold to mild, semiarid; discontinuous permafrost | Wetland; some herb, lichen tundra, evergreen forest | 353,364 |
| Montane Cordillera | Mountains and interior plains | Moderately cold, moist to arid | Evergreen forest, alpine tundra, interior grassland | 479,057 |
| Pacific Maritime | Mountains, minor coastal plains | Mild, temperate, very wet to cold alpine | Coastal evergreen forest | 205,175 |
| Southern Arctic | Plains, hills | Cold, dry; continuous permafrost | Shrub-herb tundra | 773,041 |
| Subhumid Prairies | Plains, some hills | Cold, semiarid | Grass; scattered deciduous forest (“aspen parkland”) | 469,681 |
| Taiga Cordillera | Mountains | Cold, semiarid; discontinuous permafrost | Shrub-herb-moss-lichen tundra | 264,480 |
| Taiga Plains | Plains, some foothills | Cold, semiarid to moist; discontinous permafrost | Open to closed mixed evergreen-deciduous forest | 580,139 |
| Taiga Shield East | Plains, some hills | Cold, moist to semiarid; discontinuous permafrost | Open evergreen-deciduous trees; some lichen-shrub tundra | 1,253,887 |
| Taiga Shield West |
2.1.3. Historical Climate Data
2.1.4. Scenarios of Future Climate
2.2. Methods
2.2.1. Predicting Future Spatial Distributions of Forest Productivity
2.2.2. Model Validation
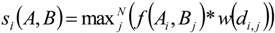
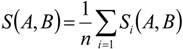
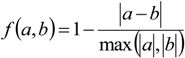
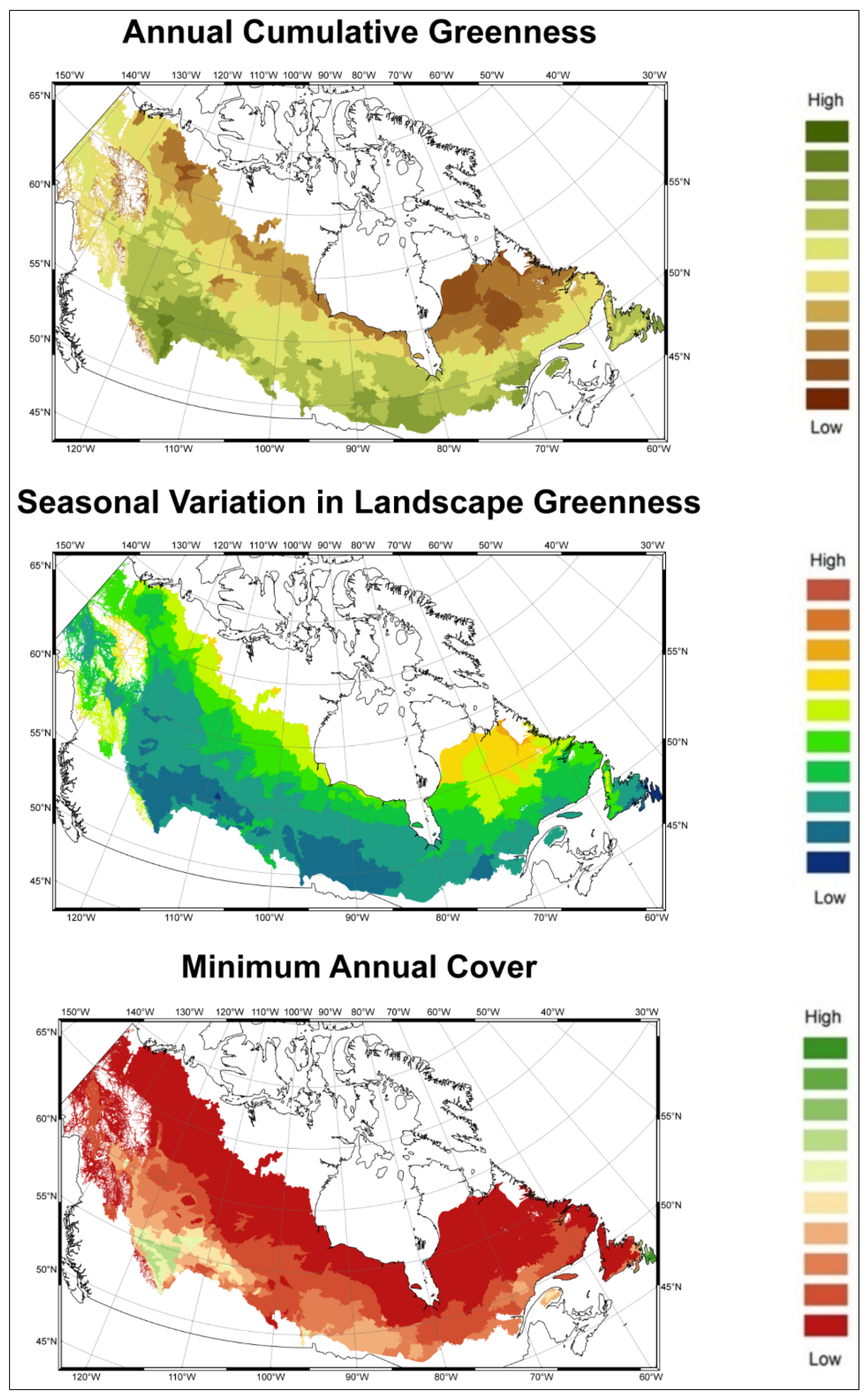
3. Results
3.1. Predicting Future Spatial Distributions of Forest Productivity
| DHI Indice | Annual Cumulative Greenness | Seasonal Variation in Greenness | Minimum Annual Cover | |||
| Variance Explained | 79.33% | 78.99% | 71.98% | |||
| Measures of Variable Importance | % Increase MSE | Increases in Node Purity | % Increase MSE | Increases in Node Purity | % Increase MSE | Increases in Node Purity |
| Ecozone ID | 74.12 | 5,169,808,231 | 74.44 | 19,389,848 | 37.81 | 1,824,596 |
| prec | 164.57 | 4,935,705,165 | 103.57 | 34,512,265 | 131.05 | 3,506,894 |
| tmax | 68.87 | 21,549,330,333 | 53.09 | 91,421,945 | 50.26 | 9,120,574 |
| tmin | 51.55 | 11,719,222,586 | 50.66 | 52,579,917 | 36.66 | 7,040,439 |
| iVPD | 101.22 | 4,630,608,283 | 78.10 | 38,235,056 | 77.19 | 34,769,256 |
| iTmin | 46.03 | 14,495,094,376 | 27.33 | 102,087,167 | 48.28 | 5,269,696 |
| GSI | 67.33 | 10,606,562,711 | 45.63 | 76,972,493 | 78.43 | 4,407,121 |
3.2. Model Validation
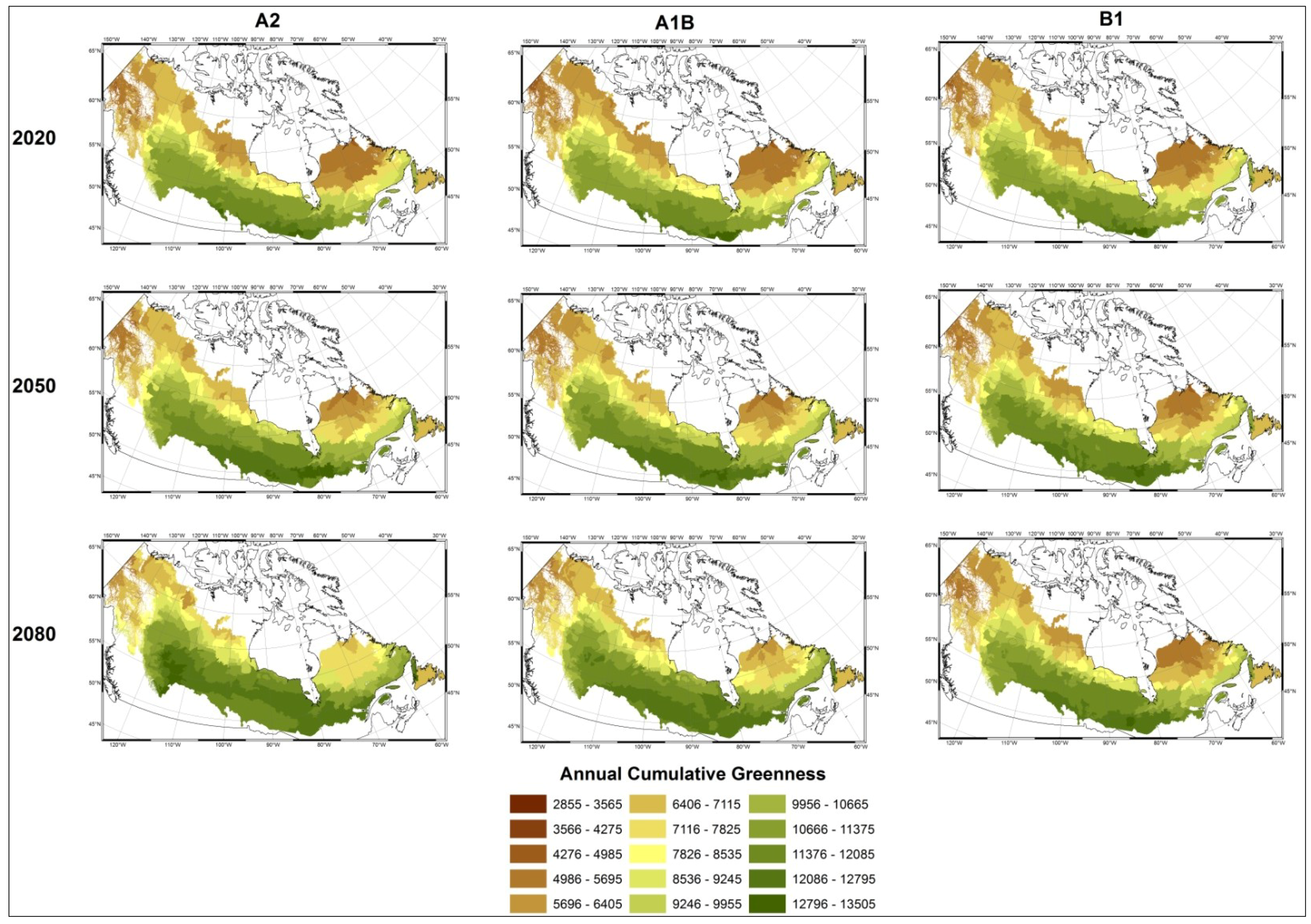
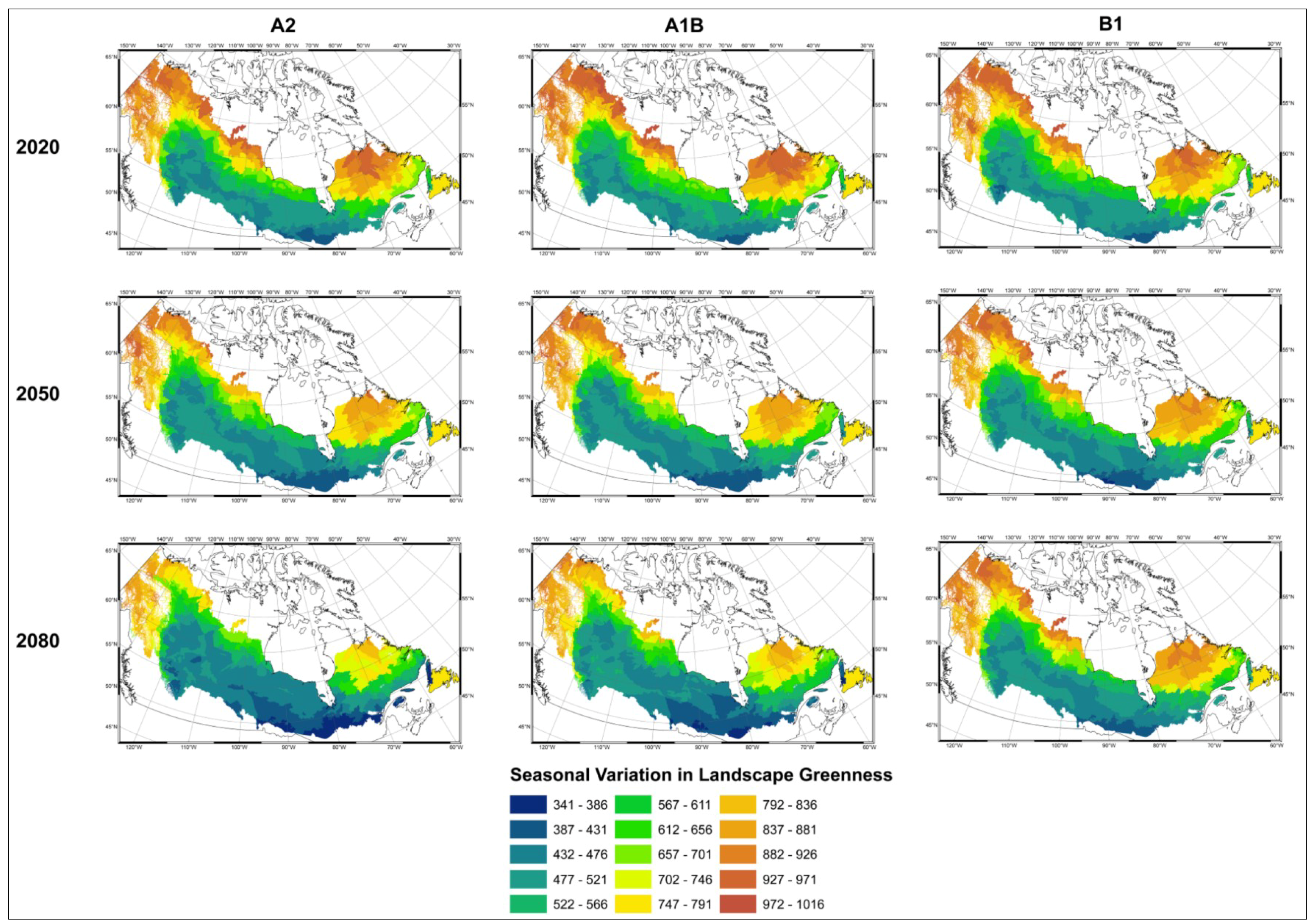

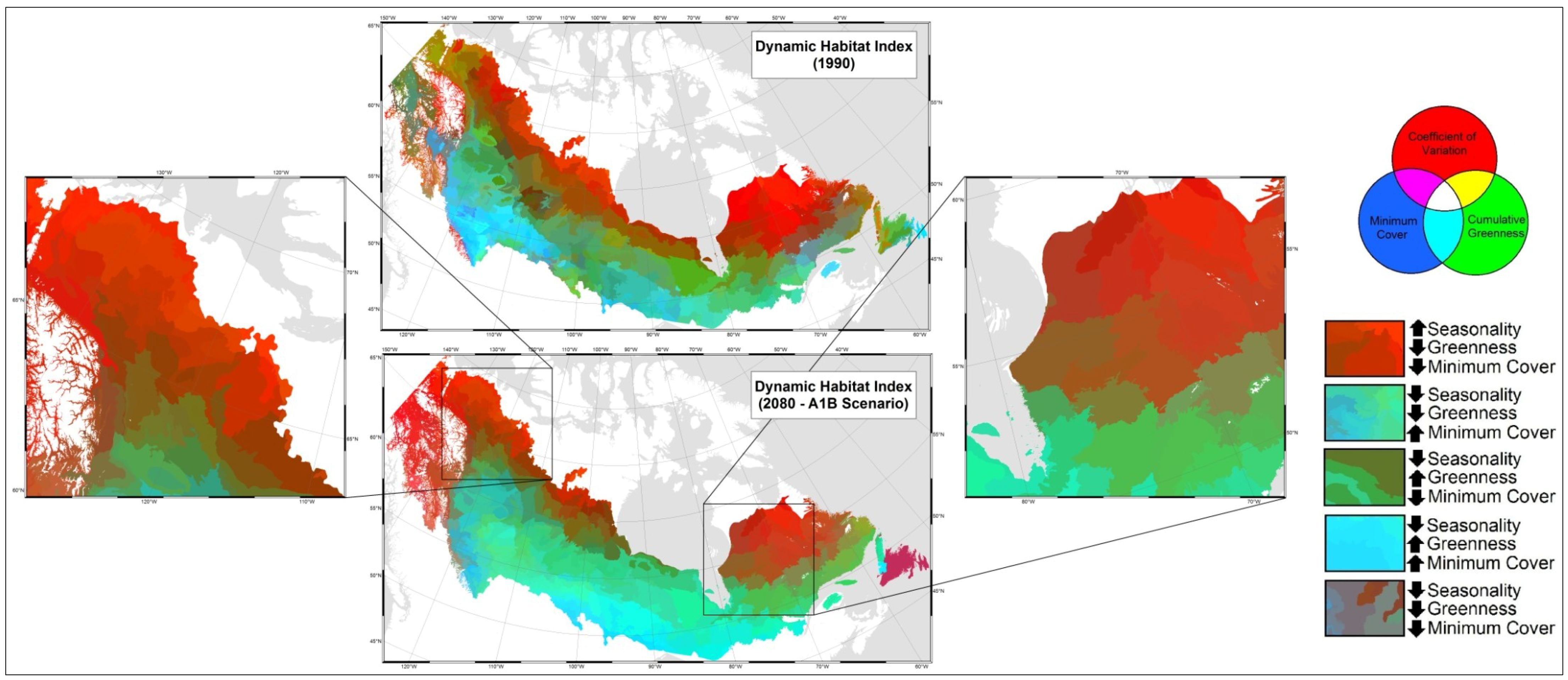
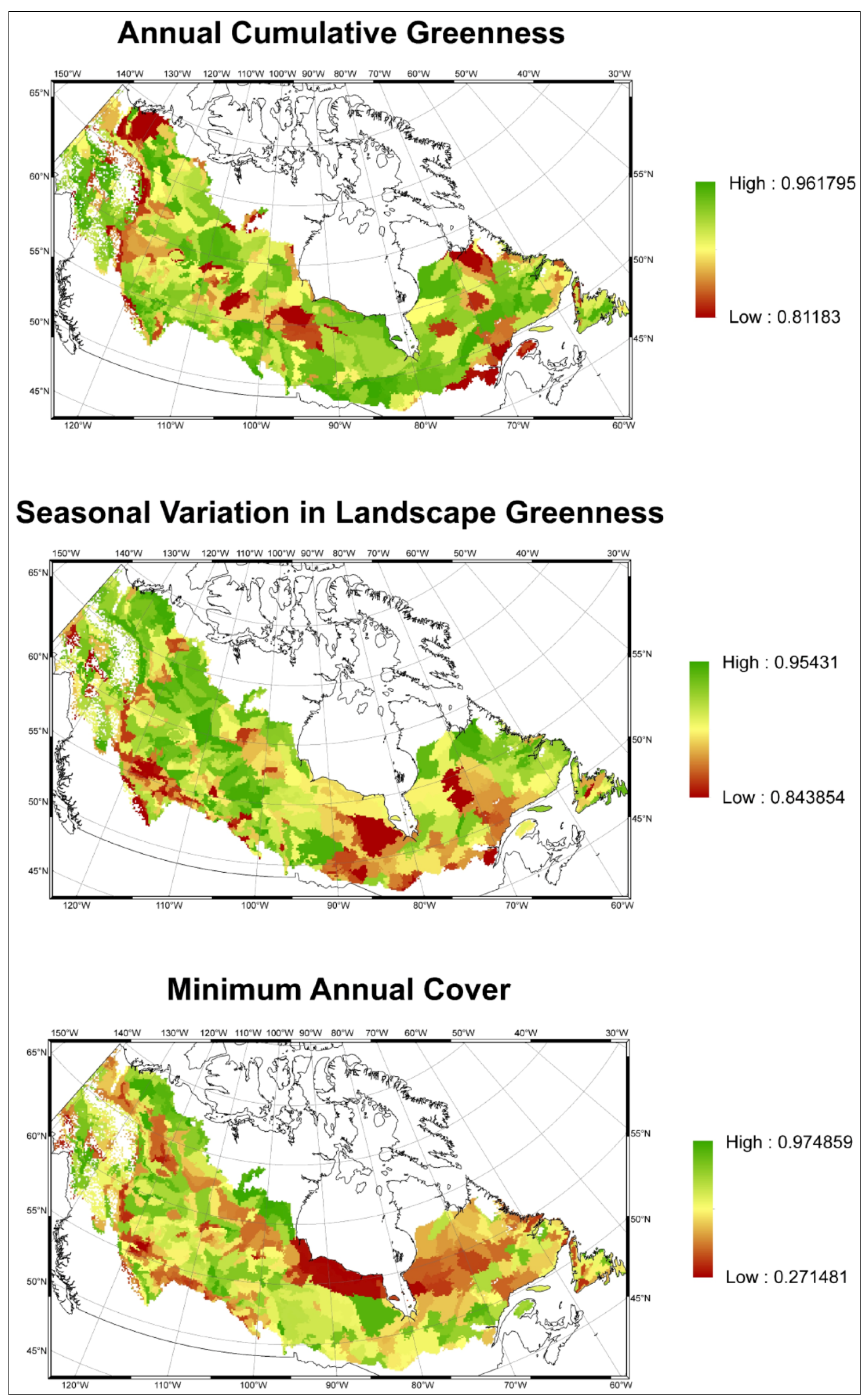
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References and Notes
- Serreze, M.C.; Walsh, J.E.; Chapin, F.S., III; Osterkamp, T.; Dyurgerov, M.; Romanovsky, V.; Oechel, W.C.; Morison, J.; Zhang, T.; Barry, R.G. Observational evidence of recent change in the northern high-latitude environment. Clim. Change 2000, 46, 159–207. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef]
- Coops, N.C.; Wulder, M.A.; Duro, D.; Han, T.; Berry, S. The development of a Canadian dynamic habitat index using multi-temporal satellite estimates of canopy light absorbance. Ecol. Indic. 2008, 8, 754–766. [Google Scholar] [CrossRef]
- Weiss, J.L.; Castro, C.L.; Overpeck, J.T. Distinguishing pronounced droughts in the southwestern United States: Seasonality and effects of warmer temperatures. J. Clim. 2009, 22, 5918–5932. [Google Scholar] [CrossRef]
- Rinawati, F.; Stein, K.; Lindner, A. Climate change impacts on biodiversity—the setting of a lingering global crisis. Diversity 2013, 5, 114–123. [Google Scholar] [CrossRef]
- Soja, A.; Tchebakova, N.; French, N.; Flannigan, M.; Shugart, H.; Stocks, B.; Sukhinin, A.; Parfenova, E.; Chapin, F.; Stackhouse, P., Jr. Climate-induced boreal forest change: Predictions versus current observations. Global Planet. Change 2007, 56, 274–296. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agr. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Wulder, M.A.; White, J.C.; Han, T.; Coops, N.C.; Cardille, J.A.; Holland, T.; Grills, D. Monitoring Canada’s forests. Part 2: National forest fragmentation and pattern. Can. J. Rem. Sens. 2008, 34, 563–584. [Google Scholar] [CrossRef]
- Geider, R.J.; Delucia, E.H.; Falkowski, P.G.; Finzi, A.C.; Grime, J.P.; Grace, J.; Kana, T.; la Roche, J.; Long, S.P.; Osborne, B.A.; et al. Primary productivity of planet earth: Biological determinants and physical constraints in terrestrial and aquatic habitats. Global Change Biol. 2001, 7, 849–882. [Google Scholar] [CrossRef]
- Soulé, M.E.; Estes, J.A.; Miller, B.; Honnold, D.L. Strongly interacting species: Conservation policy, management, and ethics. Bioscience 2005, 55, 168–176. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary response to rapid climate change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef]
- Smith, W.; Lee, P. Canada’s Forests at a Crossroads: An Assessment in the Year 2000; World Resources Institute: Washington, DC, USA, 2000. [Google Scholar]
- Andrew, M.E.; Wulder, M.A.; Coops, N.C. Identification of de facto protected areas in boreal Canada. Biol. Conserv. 2012, 146, 97–107. [Google Scholar] [CrossRef]
- Bolton, D.K.; Coops, N.C.; Wulder, M.A. Measuring forest structure along productivity gradients in the Canadian boreal with small-footprint Lidar. Environ. Monitor. Assess. 2013, 185, 6617–6634. [Google Scholar] [CrossRef]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Wright, D.H. Species-energy theory: An extension of species-area theory. Oikos 1983, 41, 496–506. [Google Scholar] [CrossRef]
- Berry, S.; MacKey, B.; Brown, T. Potential applications of remotely sensed vegetation greenness to habitat analysis and the conservation of dispersive fauna. Pac. Conserv. Biol. 2007, 13, 120–127. [Google Scholar]
- Hurlbert, A.H.; Haskell, J.P. The effect of energy and seasonality on avian species richness and community composition. Am Nat. 2003, 161, 83–97. [Google Scholar] [CrossRef]
- Hawkins, B.A. Summer vegetation, deglaciation and the anomalous bird diversity gradient in eastern North America. Global Ecol. Biogeogr. 2004, 13, 321–325. [Google Scholar] [CrossRef]
- Evans, K.L.; Neil, J.A.; Kevin, J. Abundance, species richness and energy availability in the North American avifauna. Global Ecol. Biogeogr. 2006, 15, 365–378. [Google Scholar]
- Coops, N.C.; Waring, R.H.; Wulder, M.A.; Pidgeon, A.M.; Radeloff, V.C. Bird diversity: A predictable function of satellite-derived estimates of seasonal variation in canopy light absorbance across the United States. J. Biogeogr. 2009, 36, 905–918. [Google Scholar] [CrossRef]
- Fitterer, J.L.; Nelson, T.A.; Coops, N.C.; Wulder, M.A. Modelling the ecosystem indicators of British Columbia using Earth observation data and terrain indices. Ecol. Indic. 2012, 20, 151–162. [Google Scholar] [CrossRef]
- Ivits, E.; Cherlet, M.; Horion, S.; Fensholt, R. Global biogeographical pattern of ecosystem functional types derived from Earth observation data. Rem. Sens. 2013, 5, 3305–3330. [Google Scholar] [CrossRef]
- Powers, R.P.; Coops, N.C.; Morgan, J.L.; Wulder, M.A.; Nelson, T.A.; Drever, C.R.; Cumming, S.G. A remote sensing approach to biodiversity assessment and regionalization of the Canadian boreal forest. Prog. Phys. Geog. 2013, 37, 36–62. [Google Scholar] [CrossRef]
- Fitterer, J.L.; Nelson, T.A.; Coops, N.C.; Wulder, M.A.; Mahony, N. Exploring the ecological processes driving geographical patterns of breeding bird richness in British Columbia Canada. Ecol. Appl. 2013, 23, 888–903. [Google Scholar] [CrossRef]
- Andrew, M.E.; Wulder, M.A.; Coops, N.C. Patterns of protection and threats along productivity gradients in Canada. Biol. Conserv. 2011, 144, 2891–2901. [Google Scholar] [CrossRef]
- Fontana, F.M.; Coops, N.C.; Khlopenkov, K.V.; Trishchenko, A.P.; Riffler, M.; Wulder, M.A. Generation of a novel 1 km NDVI data set over Canada, the northern United States, and Greenland based on historical AVHRR data. Rem. Sens. Environ. 2012, 121, 171–185. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Field, R.; Hawkins, B.A.; Cornell, H.V.; Currie, D.J.; Diniz-Filho, J.A.F.; Guégan, J.-F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; et al. Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 2009, 36, 132–147. [Google Scholar] [CrossRef]
- Dallmeier, F.; Comiskey, J. (Eds.) Forest Biodiversity Research, Monitoring and Modeling: Conceptual Background and Old World Case Studies; The Parthenon Publishing Group: Pearl River, NY, USA, 1998.
- Chase, J.M.; Leibold, M.A. Spatial scale dictates the productivity-biodiversity relationship. Nature 2002, 416, 427–430. [Google Scholar] [CrossRef]
- Kerr, J.T.; Southwood, T.R.; Cihlar, J. Remotely sensed habitat diversity predicts butterfly species richness and community similarity in Canada. Proc. Nat. Acad. Sci. USA. 2001, 98, 11365–11370. [Google Scholar] [CrossRef]
- Nagendra, H. Using remote sensing to assess biodiversity. Int. J. Remote Sens. 2001, 22, 2377–2400. [Google Scholar] [CrossRef]
- Turner, W.; Spector, S.; Gardiner, N.; Fledeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Nilsen, E.B.; Herfindal, I.; Linnell, J.D.C. Can intra-specific variation in carnivore home-range size be explained using remote-sensing estimates of environmental productivity? Ecoscience 2005, 12, 68–75. [Google Scholar] [CrossRef]
- Duro, D.; Coops, N.C.; Wulder, M.A.; Han, T. Development of a large area biodiversity monitoring system driven by remote sensing. Progr. Phys. Geogr. 2007, 31, 235–260. [Google Scholar] [CrossRef]
- Holmes, K.; Nelson, T.; Coops, N.C.; Wulder, M.A. Biodiversity indicators show climate change will alter vegetation in parks and protected areas. Diversity 2013, 5, 352–373. [Google Scholar] [CrossRef]
- Kurz, W.A.; Stinson, G.; Rampley, G. Could increased boreal forest ecosystem productivity offset carbon losses from increased disturbances? Philos. T. R. Soc. B 2008, 363, 2259–2268. [Google Scholar] [CrossRef]
- Brandt, J.P. The extent of the North American boreal zone. Environ. Rev. 2009, 17, 101–161. [Google Scholar] [CrossRef]
- Amiro, B.D.; Cantin, A.; Flannigan, M.D.; de Groot, W.J. Future emissions from Canadian boreal forest fires. Can. J. Forest Res. 2009, 39, 383–395. [Google Scholar] [CrossRef]
- Gralewicz, N.J.; Nelson, T.A.; Wulder, M.A. Factors influencing national scale wildfire susceptibility in Canada. Forest Ecol. Manage. 2012, 265, 20–29. [Google Scholar] [CrossRef]
- Gralewicz, N.J.; Nelson, T.A.; Wulder, M.A. Spatial and temporal patterns of wildfire ignitions in Canada from 1980 to 2006. Int. J. Wildl. Fire 2012, 21, 230–242. [Google Scholar] [CrossRef]
- Bergeron, Y.; Fenton, N.J. Boreal forests of eastern Canada revisited: Old growth, nonfire disturbances, forest succession, and biodiversity. Botany 2012, 90, 509–523. [Google Scholar] [CrossRef]
- Robertson, C.; Farmer, C.J.; Nelson, T.A.; Mackenzie, I.; Wulder, M.A.; White, J.C. Determination of the compositional change (1999–2006) in the pine forests of British Columbia due to mountain pine beetle infestation. Environ. Monitor. Assess. 2009, 158, 593–608. [Google Scholar] [CrossRef]
- Robertson, C.; Nelson, T.A.; Jelinski, D.E.; Wulder, M.A.; Boots, B. Spatial-temporal analysis of species’ range expansion: the case of the mountain pine beetle, Dendroctonus ponderosae. J. Biogeogr. 2009, 36, 1446–1458. [Google Scholar] [CrossRef]
- Rowe, J.S.; Sheard, J.W. Ecological land classification: a survey approach. Environ. Manage. 1981, 5, 451–464. [Google Scholar] [CrossRef]
- Stinson, G.; Kurz, W.A.; Smyth, C.E.; Neilson, E.T.; Dymond, C.C.; Metsaranta, J.M.; Blain, D. An inventory-based analysis of Canada’s managed forest carbon dynamics, 1990 to 2008. Global Change Biol. 2011, 17, 2227–2244. [Google Scholar] [CrossRef]
- NRTEE (National Round Table on the Environment and the Economy). Boreal Futures: Governance, Conservation and Development in Canada’s Boreal; Renouf Publishing Company Ltd.: Ottawa, Canada, 2005. Available online: http://www.stakeholderforum.org/fileadmin/files/boreal-futures-eng.pdf (accessed on 21 February 2013).
- Asrar, G.; Fuchs, M.; Kanemasu, E.T.; Hatfield, J.L. Estimating absorbed photosynthetic radiation and leaf area index from spectral reflectance in wheat. Agron. J. 1984, 76, 300–306. [Google Scholar] [CrossRef]
- Xiao, J.; Moody, A. Geographical distribution of global greening trends and their climatic correlates: 1982–1998. Int. J. Remote Sens. 2005, 26, 2371–2390. [Google Scholar] [CrossRef]
- Mesinger, F.; DiMego, G.; Kalnay, E.; Mitchell, K.; Shafran, P.C.; Ebisuzaki, W.; Jović, D.; Woollen, J.; Rogers, E.; Berbery, E.H.; et al. North American regional reanalysis. Bull. Am. Meteorol. Soc. 2006, 87, 343–360. [Google Scholar] [CrossRef]
- Jolly, W.M.; Nemani, R.; Running, S.W. A generalized, bioclimatic index to predict foliar phenology in response to climate. Global Change Biol. 2005, 11, 619–632. [Google Scholar] [CrossRef]
- Stöckli, R.; Rutishauser, T.; Baker, I.; Liniger, M.A.; Denning, A.S. A global reanalysis of vegetation phenology. J. Geophys. Res. 2011, 116, 1–19. [Google Scholar]
- Salah, H.B.H.; Tardieu, F. Quantitative analysis of the combined effects of temperature, evaporative demand and light on leaf elongation rate in well-watered field and laboratory-grown maize plants. J. Exp. Bot. 1996, 47, 1689–1698. [Google Scholar] [CrossRef]
- Cleary, B.D.; Waring, R.H. Temperature: Collection of data and its analysis for the interpretation of plant growth and distribution. Can. J. Bot. 1969, 47, 167–173. [Google Scholar] [CrossRef]
- Murdock, T.Q.; Spittlehouse, D.L. Selecting and Using Climate Change Scenarios for British Columbia; Pacific Climate Impacts Consortium: Victoria, BC, Canada, 2011; p. 39. [Google Scholar]
- Mote, P.; Salathé, E.; Peacock, C. Scenarios of Future Climate for the Pacific Northwest. Available online: http://cses.washington.edu/db/pdf/kc05scenarios462.pdf (Accessed on 21 February 2014).
- Breiman, L.E.O. Random forests. Machine Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Prasad, A.; Iverson, L.; Liaw, A. Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Archer, K.J.; Kimes, R.V. Empirical characterization of random forest variable importance measures. Computation. Stat. Data Anal. 2008, 52, 2249–2260. [Google Scholar] [CrossRef]
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psych. Meth. 2009, 14, 323–348. [Google Scholar] [CrossRef]
- Hagen-Zanker, A. Comparing Continuous Valued Raster Data: A Cross Disciplinary Literature Scan; Research Institute for Knowledge Systems: Maastricht, The Netherlands, 2006. [Google Scholar]
- Long, J.A.; Nelson, T.A.; Wulder, M.A. Local indicators for categorical data (LICD): Impacts of scaling decisions. Can. Geogr. 2010, 54, 15–28. [Google Scholar] [CrossRef]
- Nelson, T.; Boots, B.; Wulder, M.A. Techniques for accuracy assessment of tree locations extracted from remotely sensed imagery. J. Environ. Manage. 2005, 74, 265–271. [Google Scholar] [CrossRef]
- Liu, Z.; Notaro, M.; Kutzbach, J. Assessing global vegetation-climate feedbacks from observations. J. Climate 2006, 19, 787–814. [Google Scholar] [CrossRef]
- Notaro, M.; Liu, Z.; Williams, J.W. Observed vegetation–climate feedbacks in the United States. J. Climate 2006, 19, 763–786. [Google Scholar] [CrossRef]
- Pavlic, G.; Chen, W.; Fernandes, R.; Cihlar, J.; Price, D.T.; Latifovic, R.; Fraser, R.; Leblanc, S.G. Canada-wide maps of dominant tree species from remotely sensed and ground data. Geocarto Int. 2007, 22, 185–207. [Google Scholar] [CrossRef]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Flannigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakusta, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating consequences of climate change for Canada’s boreal forest ecosystems. Environ. Rev. 2013, 21, 322–365. [Google Scholar] [CrossRef]
- Wiken, E.B. Terrestrial Ecozones of Canada; Environment Canada, Lands Directorate: Ottawa, Canada, 1986. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). Third Assessment Report of the Intergovernmental Panel on Climate Change, WG I & II. Available online: http://www.grida.no/publications/other/ipcc_tar/ (accessed on 21 February 2014).
- Coops, N.C.; Wulder, M.A.; Iwanicka, D. An environmental domain classification of Canada using earth observation data for biodiversity assessment. Ecol. Inform. 2009, 4, 8–22. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetlands Ecol. Manage. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Lemmen, D.S.; Warren, F.J. (Eds.) Climate Change Impacts and Adaptation: A Canadian Perspective; Natural Resources Canada: Ottawa, ON, Canada, 2004.
- Lenihan, J.M.; Neilson, R.P. Canadian vegetation sensitivity to projected climatic change at three organizational levels. Clim. Change 1995, 30, 27–56. [Google Scholar] [CrossRef]
- Drever, M.C.; Clark, R.G.; Derksen, C.; Slattery, S.M.; Toose, P.; Nudds, T.D. Population vulnerability to climate change linked to timing of breeding in boreal ducks. Global Change Biol. 2012, 18, 480–492. [Google Scholar] [CrossRef]
- Olthof, I.; Pouliot, D. Treeline vegetation composition and change in Canada’s western subarctic from AVHRR and canopy reflectance modeling. Remote Sens. Environ. 2010, 114, 805–815. [Google Scholar] [CrossRef]
- Kerr, J.T.; Packer, L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 1997, 385, 252–254. [Google Scholar] [CrossRef]
- COSEWIC (Committee on the Status of Endangered Wildlife in Canada). COSEWIC Assessment and Status Report on the Yellow Rail (Coturnicops noveboracensis) in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, Canada, 2001. Available online: http://www.sararegistry.gc.ca/virtual_sara/files/cosewic/sr_yellow_rail_1101_e.pdf (accessed on 18 March 2013).
- Coops, N.C.; Wulder, M.A.; Iwanicka, D. Exploring the relative importance of satellite-derived descriptors of production, topography and land cover for predicting breeding bird species richness over Ontario, Canada. Remote Sens. Environ. 2009, 113, 668–679. [Google Scholar] [CrossRef]
- Schneider, S.H. Abrupt non-linear climate change, irreversibility and surprise. Global Environ. Change 2004, 14, 245–258. [Google Scholar] [CrossRef]
- Lloret, F.; Escudero, A.; Iriondo, J.M.; Martínez‐Vilalta, J.; Valladares, F. Extreme climatic events and vegetation: the role of stabilizing processes. Global Change Biol. 2012, 18, 797–805. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nelson, T.A.; Coops, N.C.; Wulder, M.A.; Perez, L.; Fitterer, J.; Powers, R.; Fontana, F. Predicting Climate Change Impacts to the Canadian Boreal Forest. Diversity 2014, 6, 133-157. https://doi.org/10.3390/d6010133
Nelson TA, Coops NC, Wulder MA, Perez L, Fitterer J, Powers R, Fontana F. Predicting Climate Change Impacts to the Canadian Boreal Forest. Diversity. 2014; 6(1):133-157. https://doi.org/10.3390/d6010133
Chicago/Turabian StyleNelson, Trisalyn A., Nicholas C. Coops, Michael A. Wulder, Liliana Perez, Jessica Fitterer, Ryan Powers, and Fabio Fontana. 2014. "Predicting Climate Change Impacts to the Canadian Boreal Forest" Diversity 6, no. 1: 133-157. https://doi.org/10.3390/d6010133






