The Influence of Water Currents on Movement Patterns on Sand in the Crown-of-Thorns Seastar (Acanthaster cf. solaris)
Abstract
:1. Introduction
2. Materials and Methods
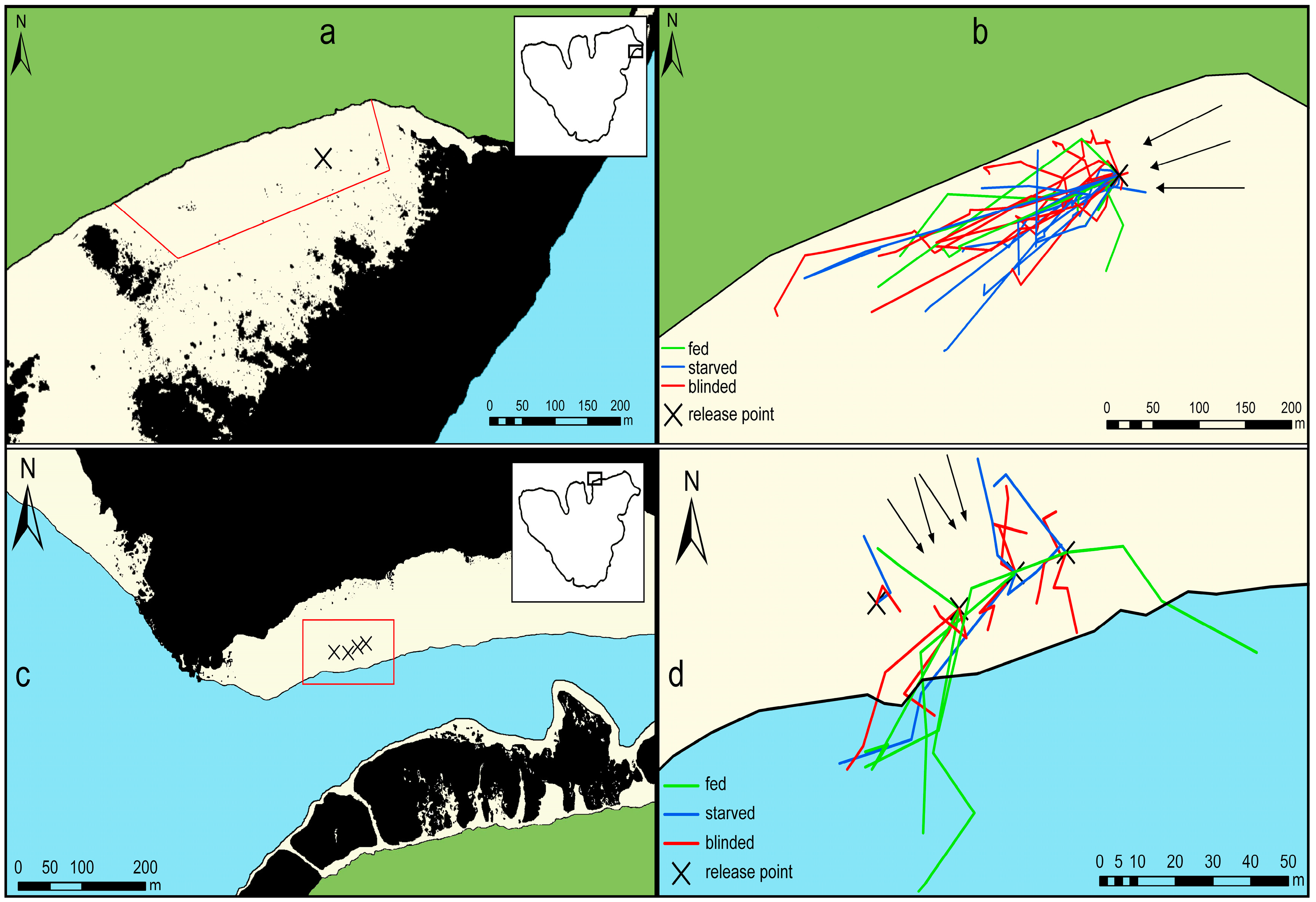
2.1. Study Site
2.2. General Experimental Procedure
2.3. Data Analysis
3. Results
3.1. Movement Patterns after One Hour
3.2. Movement Patterns after Three Hours
3.3. Movement Patterns after Several Days in Temae
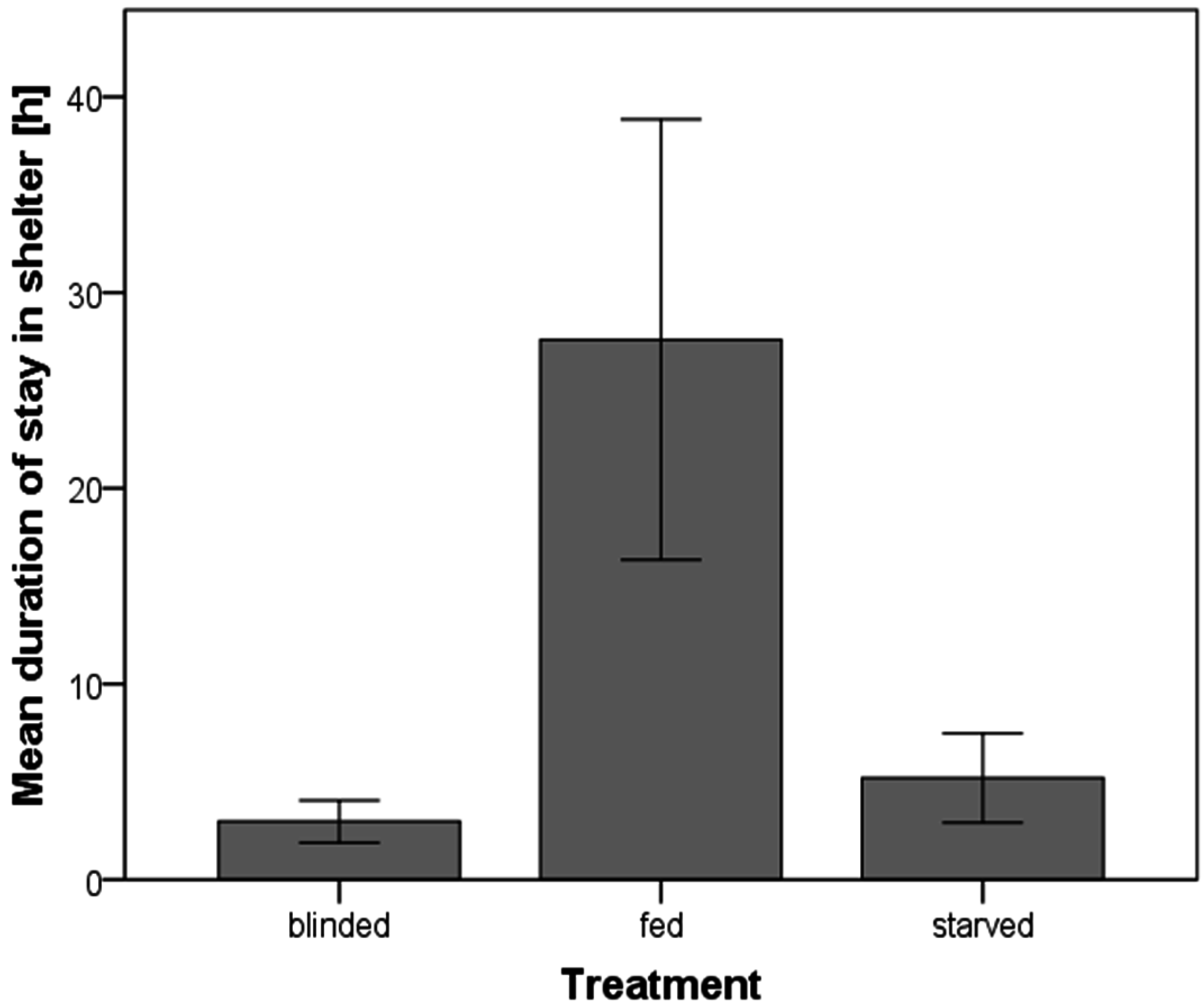
3.4. Duration of Stay in a Shelter
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster planci spp.). In Oceanography and Marine Biology: An Annual Review; Hughes, R.N., Hughes, D.J., Smith, I.P., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 133–200. [Google Scholar]
- Birkeland, C.; Lucas, J. Acanthaster Planci: Major Management Problem of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Chesher, R.H. Destruction of Pacific corals by the sea star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Endean, R. Report on Investigations Made into Aspects of the Current Acanthaster planci (crown-of-thorns) Infestations of Certain Reefs of the Great Barrier Reef. Available online: http://trove.nla.gov.au/work/24663525?q&versionId=29777393 (accessed on 29 October 2016).
- Branham, J.M.; Reed, S.; Bailey, J.H.; Caperon, J. Coral-eating sea stars Acanthaster planci in Hawaii. Science 1971, 172, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J. Preliminary observations of the decomposition of crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1992, 11, 115–118. [Google Scholar] [CrossRef]
- Branham, J.M. Crown of thorns on coral reefs. Bioscience 1973, 23, 219–226. [Google Scholar] [CrossRef]
- Kayal, M.; Vercelloni, J.; Lison de Loma, T.; Bosserelle, P.; Chancerelle, Y.; Geoffroy, S.; Stievenart, C.; Michonneau, F.; Penin, L.; Planes, S.; et al. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 2012, 7, e47363. [Google Scholar] [CrossRef] [PubMed]
- Moran, P. The Acanthaster phenomenon. Oceanogr. Mar. Biol. Annu. Rev. 1986, 24, 379–480. [Google Scholar]
- Ormond, R.F.; Campbell, A.C. Formation and breakdown of Acanthaster planci aggregations in the Red Sea. In Proceedings of the Second International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; The Great Barrier Reef Committee: Brisbane, Australia; pp. 595–619.
- Barham, E.G.; Gowdy, R.W.; Wolfson, F.H. Acanthaster (Echinodermata, Asteroidea) in the Gulf of California. Fish. Bull. 1973, 71, 927–942. [Google Scholar]
- Garm, A.; Nilsson, D. Visual navigation in starfish: First evidence for the use of vision and eyes in starfish. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Petie, R.; Hall, M.R.; Hyldahl, M.; Garm, A. Visual orientation by the crown-of-thorns starfish (Acanthaster planci). Coral Reefs 2016, 35, 1139–1150. [Google Scholar] [CrossRef]
- Sigl, R.; Steibl, S.; Laforsch, C. The role of vision for navigation in the crown-of-thorns seastar, Acanthaster planci. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.; Brauer, R.; Jordan, M. Locomotory response of Acanthaster planci to various species of coral. Nature 1970, 228, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.C.; Crisp, D.J. Responses of Asterias rubens to olfactory stimuli. J. Mar. Biol. Assoc. UK 1970, 50, 829–847. [Google Scholar] [CrossRef]
- Campbell, A.; Coppard, S.; D’Abreo, C.; Tudor-Thomas, R. Escape and aggregation responses of three echinoderms to conspecific stimuli. Biol. Bull. 2001, 201, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rochette, R.; Hamel, J.; Himmelman, J. Foraging strategy of the asteroid Leptasterias polaris: Role of prey odors, current and feeding status. Mar. Ecol. Prog. Ser. 1994, 106, 93–100. [Google Scholar] [CrossRef]
- Ormond, R.; Campbell, A.C.; Head, S.H.; Moore, R.J.; Rainbow, P.R.; Saunders, A.P. Formation and breakdown of aggregations of the crown-of-thorns starfish, Acanthaster planci (L.). Nature 1973, 246, 167–169. [Google Scholar] [CrossRef]
- Sloan, N. Aspects of the feeding biology of Asteroids. Oceanogr. Mar. Biol. Annu. Rev. 1980, 18, 57–124. [Google Scholar]
- Atema, J. Chemoreception in the sea: Adaptations of chemoreceptors and behaviour to aquatic stimulus conditions. Symp. Soc. Exp. Biol. 1985, 39, 387–423. [Google Scholar] [PubMed]
- Sloan, N.; Northway, S. Chemoreception by the Asteroid Crossaster papposus (L.). J. Exp. Mar. Biol. Ecol. 1982, 61, 85–98. [Google Scholar] [CrossRef]
- Sloan, N.; Campbell, A. Perception of food. In Echinoderm Nutrition; Jangoux, M., Lawrence, J., Eds.; Balkema: Rotterdam, The Netherlands, 1982; pp. 3–23. [Google Scholar]
- Drolet, D.; Himmelman, J.H. Role of current and prey odour in the displacement behaviour of the sea star Asterias vulgaris. Can. J. Zool. 2004, 82, 1547–1553. [Google Scholar] [CrossRef]
- Brauer, R.; Jordan, M. Triggering of the stomach eversion reflex of Acanthaster planci by coral extracts. Nature 1970, 228, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Valentinčič, T. Food finding and stimuli to feeding in the sea star Marthasterias glacialis. Neth. J. Sea Res. 1973, 7, 191–199. [Google Scholar] [CrossRef]
- Ribi, G.; Jost, P. Feeding rate and duration of daily activity of Astropecten aranciacus (Echinodermata: Asteroidea) in relation to prey density. Mar. Biol. 1978, 45, 249–254. [Google Scholar] [CrossRef]
- McClintock, J.B.; Lawrence, J.M. Ingestive conditioning in Luidia clathrata (Say) (Echinodermata: Asteroidea): Effect of nutritional condition on selectivity, teloreception, and rates of ingestion. Mar. Behav. Physiol. 1984, 10, 167–181. [Google Scholar] [CrossRef]
- McCurley, R.S.; Kier, W.M. The functional morphology of starfish tube feet: The role of a crossed-fiber helical array in movement. Biol. Bull. 1995, 188, 197–209. [Google Scholar] [CrossRef]
- Wall, J. Movement Ecology Tools for ArcGIS (ArcMET). Available online: www.movementecology.net (accessed on 12 August 2014).
- Jammalamadaka, S.R.; SenGupta, A. Topics in Circular Statistics; World Scientific Publishing: Singapore, 2001. [Google Scholar]
- Mueller, B.; Bos, A.; Graf, G.; Gumanao, G. Size-specific locomotion rate and movement pattern of four common Indo-Pacific sea stars (Echinodermata; Asteroidea). Aquat. Biol. 2011, 12, 157–164. [Google Scholar] [CrossRef]
- Scheibling, R. Optimal foraging movements of Oreaster reticulatus (L.) (Echinodermata: Asteroidea). J. Exp. Mar. Biol. Ecol. 1981, 51, 173–185. [Google Scholar] [CrossRef]
- Castilla, J.C.; Crisp, D.J. Responses of Asterias rubens to water currents and their modification by certain environmental factors. Neth. J. Sea Res. 1973, 7, 171–190. [Google Scholar] [CrossRef]
- Fenchel, T. Feeding biology of the sea-star Luidia sarsi Düben & Koren. Ophelia 1965, 2, 223–236. [Google Scholar]
- Nickell, T.D.; Moore, P.G. The behavioural ecology of epibenthic scavenging invertebrates in the Clyde Sea area: Laboratory experiments on attractions to bait in moving water, underwater TV observation in situ and general conclusions. J. Exp. Mar. Biol. Ecol. 1992, 159, 15–35. [Google Scholar] [CrossRef]
- Goreau, T.F.; Lang, J.C.; Graham, E.A. Structure and ecology of the Saipan Reefs in relation to predation by Acanthaster planci (Linnaeus). Bull. Mar. Sci. 1972, 22, 113–152. [Google Scholar]
- Montgomery, E.M.; Palmer, A.R. Effects of body size and shape on locomotion in the bat star (Patiria miniata). Biol. Bull. 2012, 222, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuizen, H.; Oakes, V. Behavioral responses of seven species of Asteroids to the Asteroid predator, Solaster dawsoni. Oecologia 1981, 48, 214–220. [Google Scholar] [CrossRef]
- McClintock, J.B.; Lawrence, J. Characteristics of foraging in the soft-bottom benthic starfish Luidia clathrata (Echinodermata: Asteroidea): Prey selectivity, switching behavior, functional responses and movement patterns. Oecologia 1985, 66, 291–298. [Google Scholar] [CrossRef]
| Site | Treatment | N | Circular Mean Direction | Mean Current Velocity [m·s−1] | Rao’s Spacing Test p-Value |
|---|---|---|---|---|---|
| Temae | blinded | 8 | 232.9° | 0.163 ± 0.076 | 0.056 |
| Temae | starved | 6 | 251.3° | 0.163 ± 0.076 | 0.026 * |
| Temae 1 | fed | 1 | 265.8° | 0.163 ± 0.076 | - |
| Maharepa | blinded | 10 | 242.0° | 0.096 ± 0.029 | 0.400 |
| Maharepa | starved | 5 | 275.9° | 0.096 ± 0.029 | 0.506 |
| Maharepa | fed | 6 | 230.7° | 0.096 ± 0.029 | 0.333 |
| Site | Treatment | N | D:Wall ± SD | Circular Mean Direction | Mean Current Velocity [m·s−1] | Rao’s Spacing Test p-Value |
|---|---|---|---|---|---|---|
| Temae | blinded | 6 | 0.83 ± 0.27 | 257.0° | 0.183 ± 0.062 | 0.004 * |
| Temae | starved | 4 | 0.89 ± 0.17 | 258.8° | 0.183 ± 0.062 | 0.011 * |
| Temae 1 | fed | 1 | 0.98 | 257.4° | 0.183 ± 0.062 | - |
| Maharepa | blinded | 10 | 0.70 ± 0.18 | 230.9° | 0.096 ± 0.029 | 0.500 |
| Maharepa | starved | 4 | 0.85 ± 0.15 | 319.7° | 0.096 ± 0.029 | 0.818 |
| Maharepa | fed | 5 | 0.79 ± 0.07 | 196.9° | 0.096 ± 0.029 | 0.074 |
| Site | Treatment | N 1st h | Mean Displacement ± SD [m] after 1 h | N 3rd h | Mean Displacement (D) ± SD [m] after 3 h |
|---|---|---|---|---|---|
| Temae | blinded | 8 | 16.7 ± 10.5 | 6 | 49.8 ± 27.7 |
| Temae | starved | 6 | 13.4 ± 5.6 | 4 | 26.3 ± 13.4 |
| Temae 1 | fed | 1 | 11.8 | 1 | 27.9 |
| Maharepa | blinded | 10 | 7.2 ± 5.7 | 10 | 15.7 ± 10.2 |
| Maharepa | starved | 5 | 12.2 ± 11.3 | 4 | 29.7 ± 17.3 |
| Maharepa | fed | 6 | 27.4 ± 7.9 | 5 | 49.8 ± 8.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigl, R.; Laforsch, C. The Influence of Water Currents on Movement Patterns on Sand in the Crown-of-Thorns Seastar (Acanthaster cf. solaris). Diversity 2016, 8, 25. https://doi.org/10.3390/d8040025
Sigl R, Laforsch C. The Influence of Water Currents on Movement Patterns on Sand in the Crown-of-Thorns Seastar (Acanthaster cf. solaris). Diversity. 2016; 8(4):25. https://doi.org/10.3390/d8040025
Chicago/Turabian StyleSigl, Robert, and Christian Laforsch. 2016. "The Influence of Water Currents on Movement Patterns on Sand in the Crown-of-Thorns Seastar (Acanthaster cf. solaris)" Diversity 8, no. 4: 25. https://doi.org/10.3390/d8040025






