Classification of Alzheimer’s Patients through Ubiquitous Computing †
Abstract
:1. Motivation and Research Context
- The patient and/or carer that turns on and off the accelerometer of the smartphone in order for it to save and analyze the data.
- The doctor that receives the analysis of the data and that has to take the decision to whether make a more thorough examination in a control ambient with other techniques.
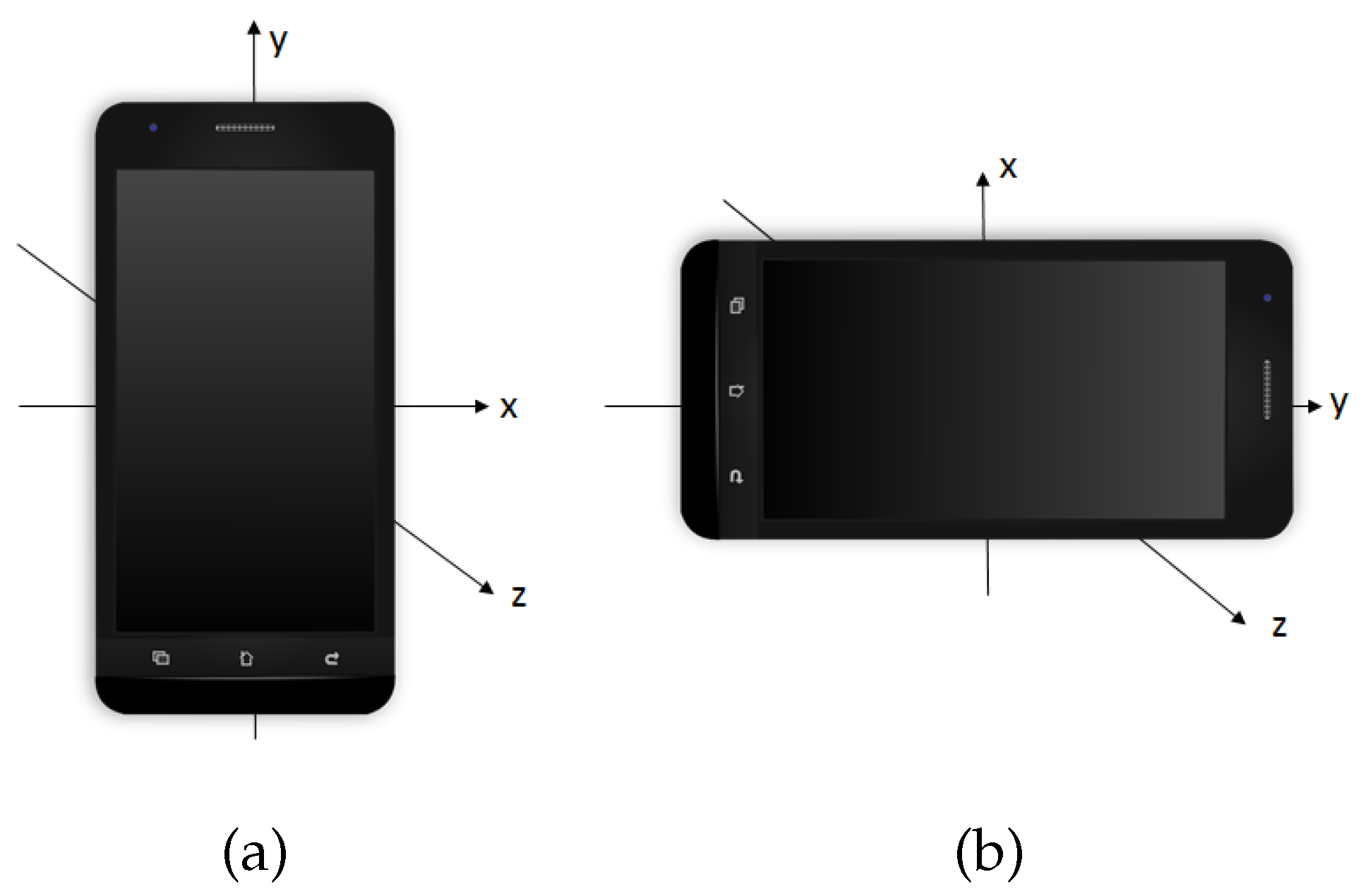
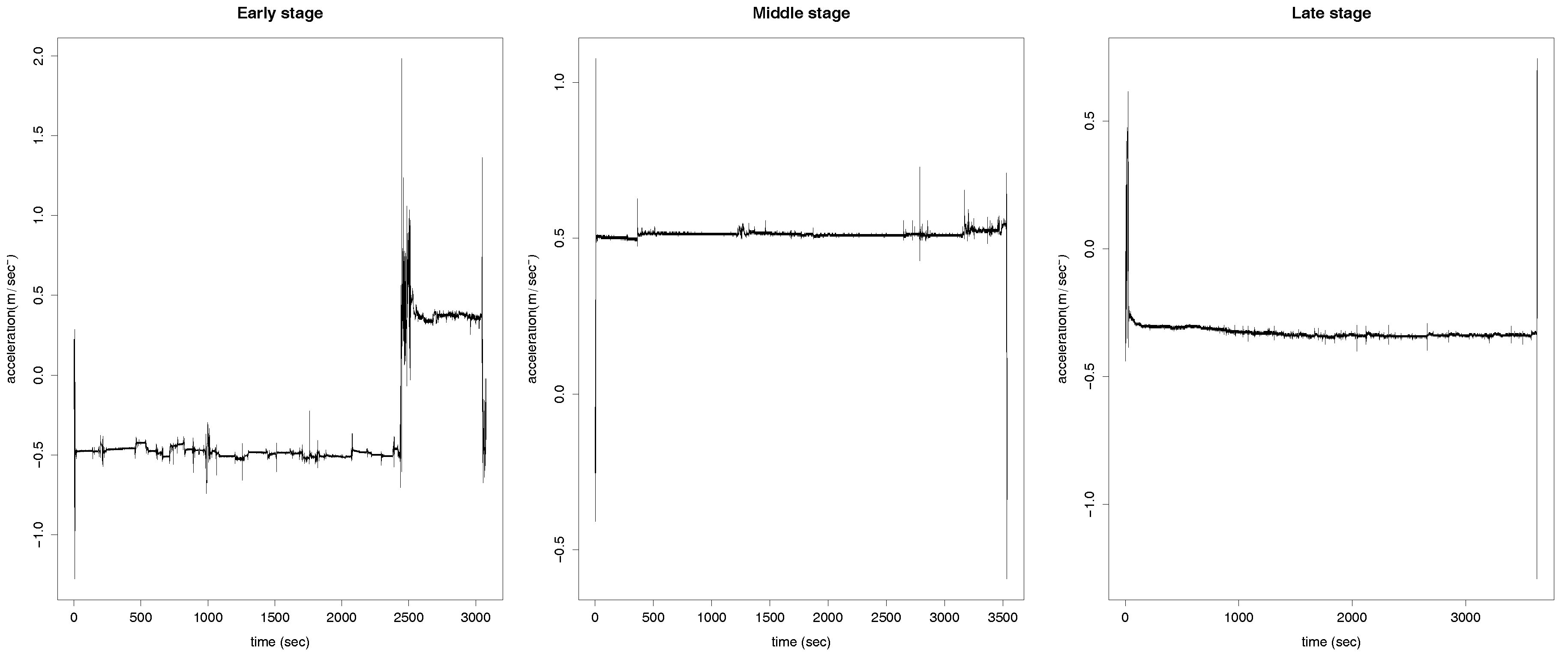
2. Real Data
3. Functional Data Analysis
3.1. Functional Analysis of Variance
3.2. Results of Applying the Tests to the Real Data
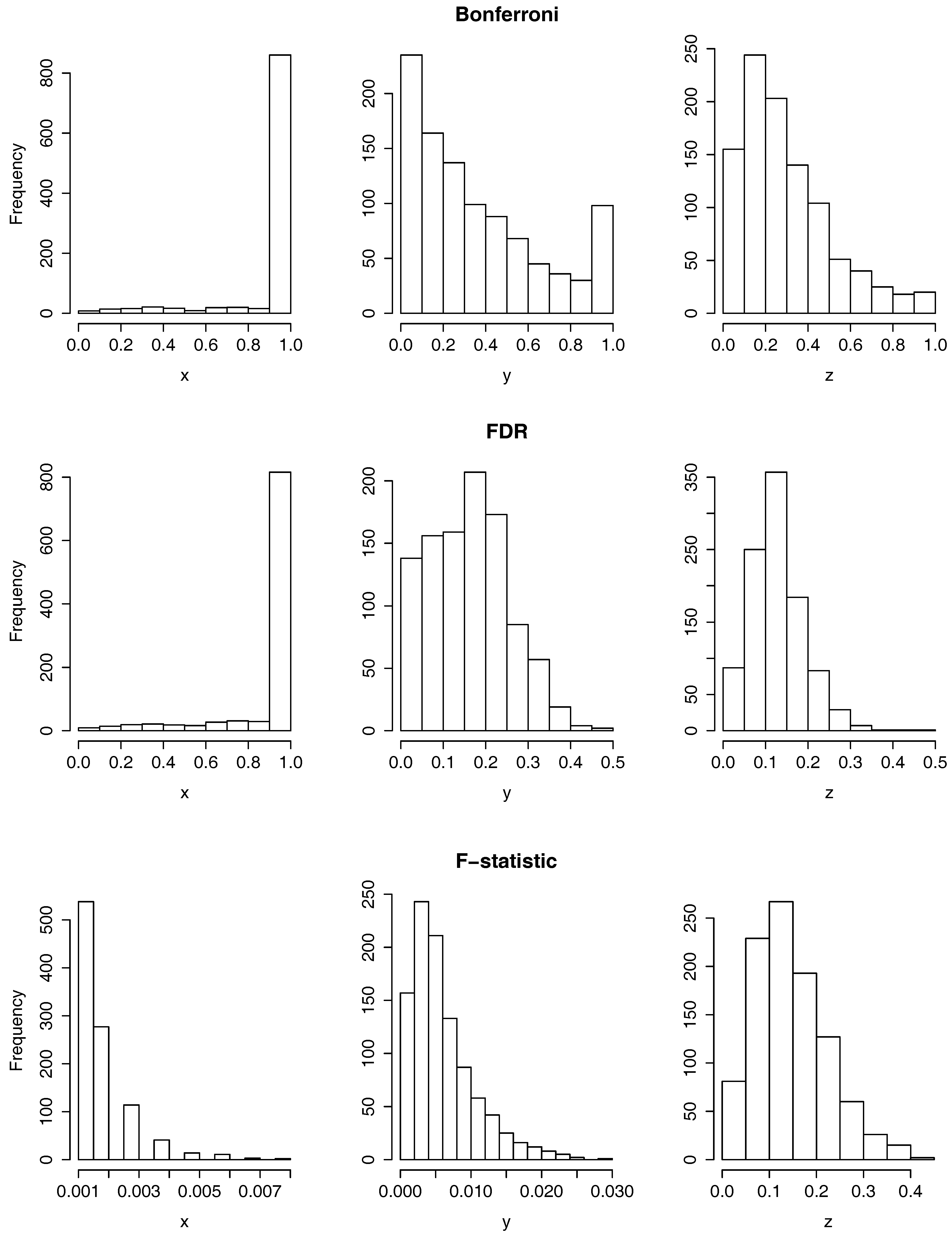
3.2.1. Results of Applying the ANOVA Tests
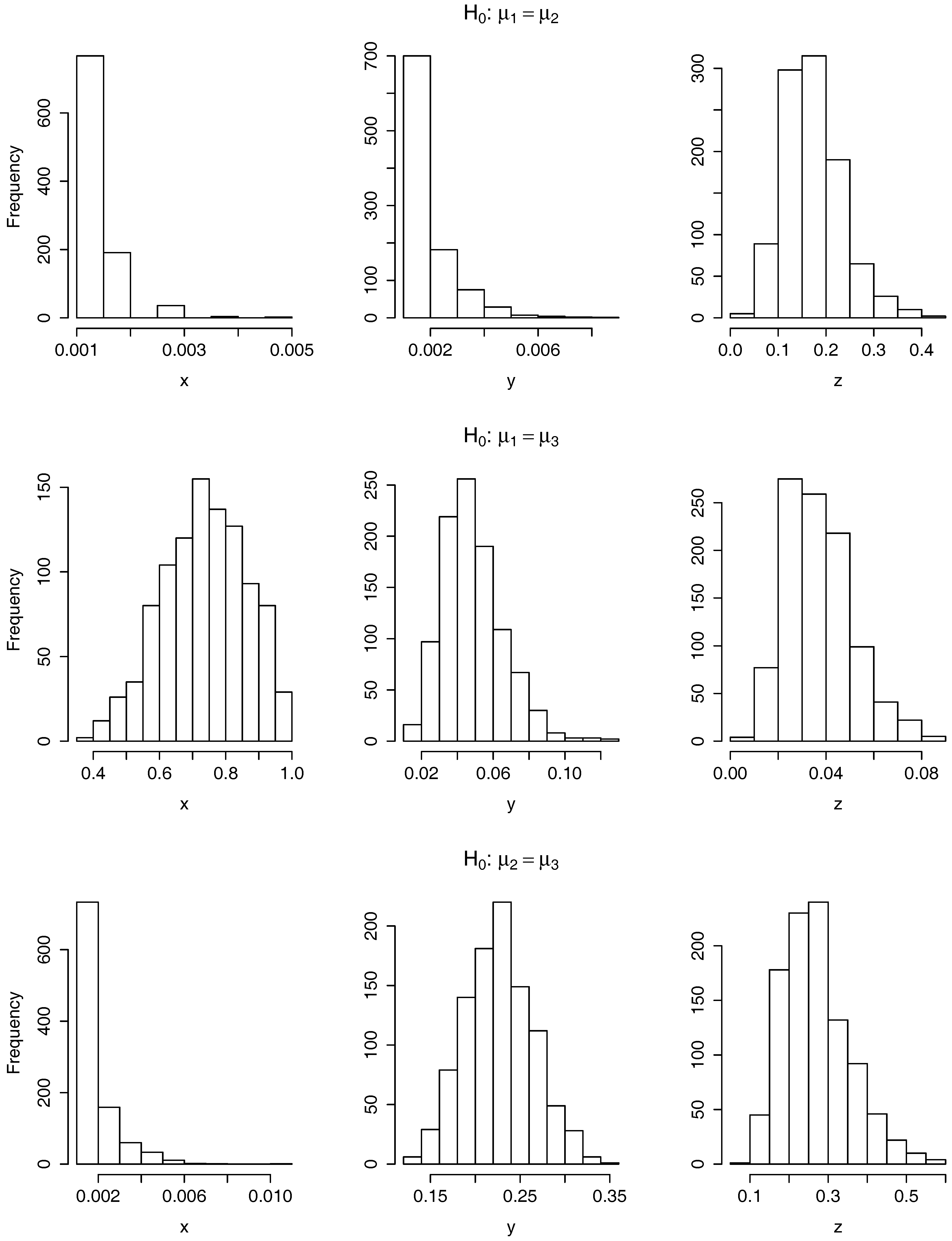
3.2.2. Results of Applying the Equality of Means Tests
3.2.3. Conclusions on the Results of Applying the Tests
- The patients in the early stage of their disease from the middle stage because of the x and y-axis.
- The patients in the middle stage of their disease from the late stage because of the x-axis.
- The patients in the early stage of their disease from the late stage because of the y and z-axis.
3.3. Functional Supervised Classification
4. Supervised Learning Classifiers
4.1. Filtering Features
4.2. Neural Networks Versus other Classifiers
5. Related Work
6. Conclusions
- There is a range of observed three-dimensional functional data per patient, which varies between two and eight.
- Each functional datum is observed in a different grid, with a different frequency of observations, even when the data belong to the same patient.
- Each functional datum is observed in a domain of different length.
- Hypothesis testing on the equality of the means of groups of patients on different stages of the disease to find differences on the accelerations patterns among different stages of the disease.
- Functional supervised classification to classify the patients according to their set of observed acceleration curves into their stage of the disease.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiser, M. The Computer for the 21st Century. SIGMOBILE Mob. Comput. Commun. Rev. 1999, 3, 3–11. [Google Scholar] [CrossRef]
- Acampora, G.; Cook, D.J.; Rashidi, P.; Vasilakos, A.V. A Survey on Ambient Intelligence in Healthcare. Proc. IEEE 2013, 101, 2470–2494. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.; de Leon, M.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [PubMed]
- IJmker, T.; Lamoth, C.J. Gait and cognition: The relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture 2012, 35, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Albertos, J.A.; Febrero-Bande, M. A simple multiway ANOVA for functional data. TEST 2010, 19, 537–557. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, M.; Tseng, C.; Wu, H.T. A New Test for One-Way ANOVA with Functional Data and Application to Ischemic Heart Screening. Available online: http://arxiv.org/abs/1309.7376 (accessed on 12 January 2017).
- Benko, M.; Hardle, W.; Kneip, A. Common functional principal components. Ann. Stat. 2009, 37, 1–34. [Google Scholar] [CrossRef]
- Cuesta-Albertos, J.A.; Febrero-Bande, M.; Oviedo de la Fuente, M. The DDG-classifier in the functional setting. TEST 2017, 26, 119–142. [Google Scholar] [CrossRef]
- Nieto-Reyes, A.; Battey, H. A topologically valid definition of depth for functional data. Stat. Sci. 2016, 31, 61–79. [Google Scholar] [CrossRef]
- Cuevas, A.; Febrero, M.; Fraiman, R. Robust estimation and classification for functional data via projection-based depth notions. Comput. Stat. 2007, 22, 481–496. [Google Scholar] [CrossRef]
- Kasabov, N.K. Foundations of Neural Networks, Fuzzy Systems, and Knowledge Engineering, 1st ed.; MIT Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Sakurai, R.; Ishii, K.; Yasunaga, M.; Takeuchi, R.; Murayama, Y.; Sakuma, N.; Sakata, M.; Oda, K.; Ishibashi, K.; Ishiwata, K.; et al. The neural substrate of gait and executive function relationship in elderly women: A PET study. Geriatr. Gerontol. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Meguro, K.; Yamazaki, H.; Okuzumi, H.; Tanaka, A.; Horikawa, A.; Yamaguchi, K.; Katsuyama, N.; Nakano, M.; Aral, H.; et al. Postural and Gait Disturbance Correlated with Decreased Frontal Cerebral Blood Flow in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 1997, 11, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Annweiler, C.; Callisaya, M.L.; Cock, A.M.D.; Helbostad, J.L.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.P.; Blumen, H.M.; Verghese, J.; et al. Poor Gait Performance and Prediction of Dementia: Results From a Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Annweiler, C.; Blumen, H.M.; Callisaya, M.L.; De Cock, A.M.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.P.; Verghese, J.; Beauchet, O. Gait phenotype from mild cognitive impairment to moderate dementia: Results from the GOOD initiative. Eur. J. Neurol. 2016, 23, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Gietzelt, M.; Feldwieser, F.; Gövercin, M.; Steinhagen-Thiessen, E.; Marschollek, M. A prospective field study for sensor-based identification of fall risk in older people with dementia. Inform. Health Soc. Care 2014, 39, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Gietzelt, M.; Wolf, K.H.; Kohlmann, M.; Marschollek, M.; Haux, R. Measurement of Accelerometry-based Gait Parameters in People with and without Dementia in the Field. A Technical Feasibility Study. Methods Inform. Med. 2013, 52, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Mitoma, H.; Sanjo, N.; Higuma, M.; Terashi, H.; Yokota, T. Ambulatory Gait Behavior in Patients With Dementia: A Comparison With Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Kirste, T.; Hoffmeyer, A.; Koldrack, P.; Bauer, A.; Schubert, S.; Schröder, S.; Teipel, S. Detecting the effect of Alzheimer’s disease on everyday motion behavior. J. Alzheimers Dis. 2014, 38, 121–132. [Google Scholar] [PubMed]
- Capela, N.A.; Lemaire, E.D.; Baddour, N.; Rudolf, M.; Goljar, N.; Burger, H. Evaluation of a smartphone human activity recognition application with able-bodied and stroke participants. J. NeuroEng. Rehabil. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
| Tests | ||||||
|---|---|---|---|---|---|---|
| Bonferroni | FDR | F-Statistic | ||||
| x-axis | ||||||
| mean | 0.9266 | 0.8957 | 0.0018 | 0.0013 | 0.7356 | 0.0020 |
| stand. dev. | 0.2027 | 0.2060 | 0.0011 | 0.0006 | 0.1256 | 0.0012 |
| proportion | 0.004 | 0.004 | 1 | 1 | 0 | 1 |
| y-axis | ||||||
| mean | 0.3603 | 0.1616 | 0.0066 | 0.0021 | 0.0492 | 0.2267 |
| stand. dev. | 0.3015 | 0.0931 | 0.0046 | 0.0012 | 0.0168 | 0.0381 |
| proportion | 0.128 | 0.138 | 1 | 1 | 0.588 | 0 |
| z-axis | ||||||
| mean | 0.2971 | 0.1282 | 0.1465 | 0.1733 | 0.0377 | 0.2702 |
| stand. dev. | 0.2145 | 0.0620 | 0.0762 | 0.0616 | 0.0139 | 0.0853 |
| proportion | 0.065 | 0.087 | 0.081 | 0.005 | 0.833 | 0 |
| Missclassifications | Success Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Early | Middle | Late | Total | Early | Middle | Late | Total | |
| x-axis | 7 | 2 | 9 | 18 | 0% | 89% | 10% | 49% |
| y-axis | 7 | 4 | 8 | 19 | 0% | 78% | 20% | 46% |
| -axis | 6 | 2 | 9 | 17 | 14% | 89% | 10% | 51% |
| Training Sample | Test Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Splitting | Early | Middle | Late | Total | Early | Middle | Late | Total | |||
| studied | 6 | 15 | 8 | 29 | 1 | 3 | 2 | 6 | |||
| 1 | 5 | 15 | 8 | 28 | 2 | 3 | 2 | 7 | |||
| 2 | 5 | 14 | 8 | 27 | 2 | 4 | 2 | 8 | |||
| Neural Network Parameters | Values |
|---|---|
| Package | neuralnet |
| Input neurons | 90 |
| Hidden neurons | 175 |
| Output neurons | 1 |
| Bias | 1 per hidden layer |
| Max iterations | 1000 |
| Activation function | logistic |
| Algorithm | resilient back-propagation with weight backtracking (rprop+) |
| Missclassification Rate | Success Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Technique | Early-Stage | Middle-Stage | Late-Stage | Total | Early-Stage | Middle-Stage | Late-Stage | Total |
| NN | 0 | 0 | 1 | 1 | 100% | 100% | 50% | 83% |
| RT | 1 | 0 | 2 | 3 | 0% | 100% | 0% | 50% |
| RF | 1 | 0 | 2 | 3 | 0% | 100% | 0% | 50% |
| SVM | 1 | 0 | 2 | 3 | 0% | 100% | 0% | 50% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Reyes, A.; Duque, R.; Montaña, J.L.; Lage, C. Classification of Alzheimer’s Patients through Ubiquitous Computing. Sensors 2017, 17, 1679. https://doi.org/10.3390/s17071679
Nieto-Reyes A, Duque R, Montaña JL, Lage C. Classification of Alzheimer’s Patients through Ubiquitous Computing. Sensors. 2017; 17(7):1679. https://doi.org/10.3390/s17071679
Chicago/Turabian StyleNieto-Reyes, Alicia, Rafael Duque, José Luis Montaña, and Carmen Lage. 2017. "Classification of Alzheimer’s Patients through Ubiquitous Computing" Sensors 17, no. 7: 1679. https://doi.org/10.3390/s17071679




