Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Individual Detection of Carbaryl and Paraquat
3.1.1. Cyclic Voltammetry
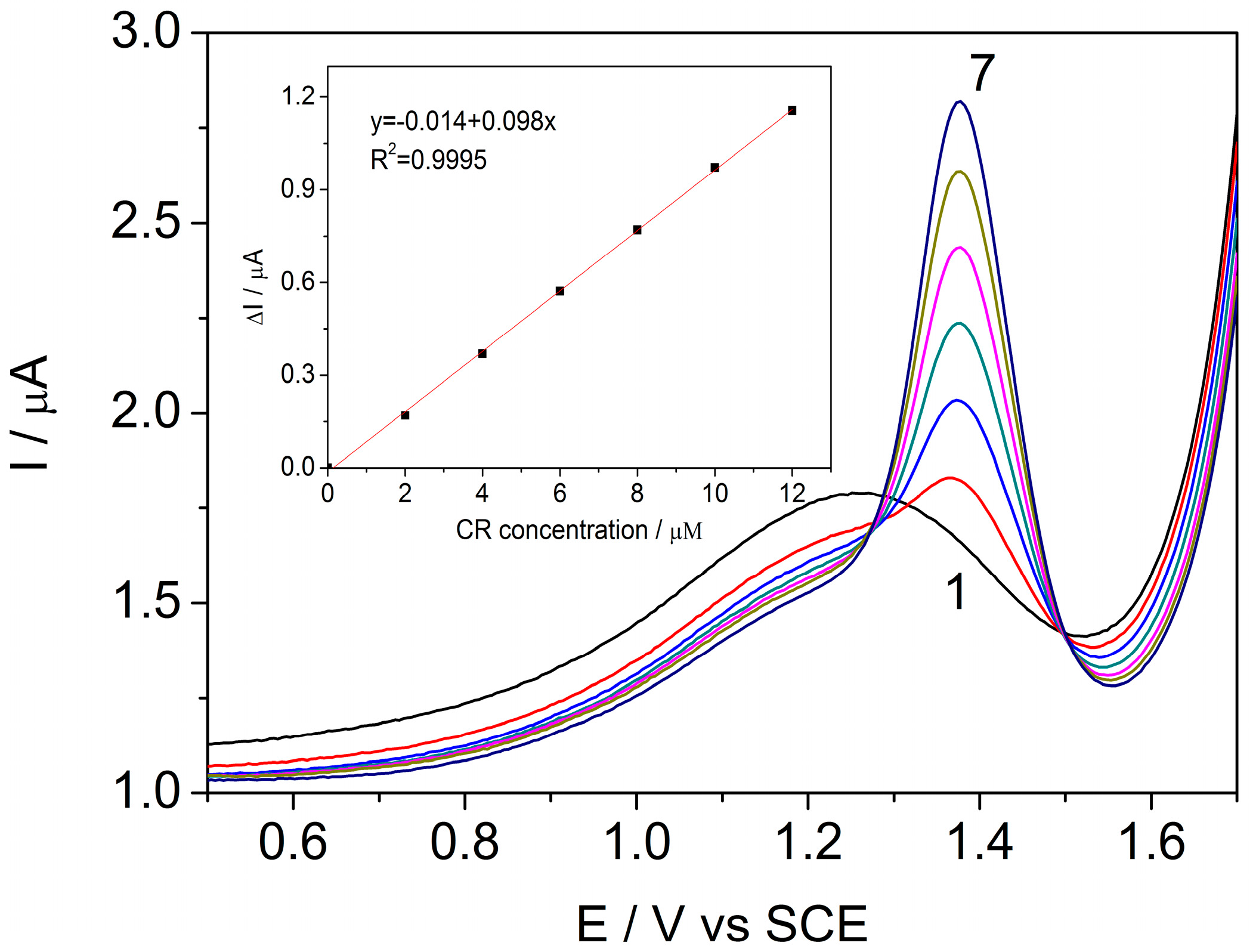
3.1.2. Differential Pulse Voltammetry
3.2. Simultaneous Detection of Carbaryl and Paraquat
3.2.1. Simultaneous Detection of CR and PQ in Acetate Buffer
3.2.2. Simultaneous Detection of CR and PQ in Natural Apple Juice
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chu, X.-G.; Hu, X.-Z.; Yao, H.Y. Determination of 266 pesticide residues in apple juice matrix solid-phase dispersion and gas chromatography-mass selective detection. J. Chromatogr. A 2005, 1063, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-H.; Cho, T.-H.; Hong, C.-K.; Kim, M.-S.; Cho, S.-J.; Park, W.-H.; Hwang, I.-S.; Kim, M.-S. Monitoring and Risk Assessment of Pesticide Residues in Commercially Dried Vegetables. Prev. Nutr. Food Sci. 2013, 18, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Masia, A.; Blasco, C.; Pico, Y. Last trends in pesticide residue determination by liquid chromatography-mass spectrometry. Trends Environ. Anal. Chem. 2014, 2, 11–24. [Google Scholar] [CrossRef]
- Souza, D.F.; Souza, E.L.; Borges, E.M. Determination of Pesticides in Grape Juices by QuEChERS and Liquid Chromatography-Tandem Mass Spectrometry. J. Braz. Chem. Soc. 2016, 27, 1626–1635. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials: A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rousis, N.I.; Bade, R.; Bijlsma, L.; Zuccato, E.; Sancho, J.V.; Hernandez, F.; Castiglioni, S. Monitoring a large number of pesticides and transformation products in water samples from Spain and Italy. Environ. Res. 2017, 156, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Du, S.; Wang, X. Amperometric immunosensor for carbofuran detection based on gold nanoparticles and PB-MWCNTs-CTS composite film. Eur. Food Res. Technol. 2012, 235, 469–477. [Google Scholar] [CrossRef]
- Sanchez-Barragan, I.; Karim, K.; Costa-Fernadez, J.M.; Piletsky, S.A.; Sanz-Medel, A. A molecularly imprinted polymer for carbaryl determination in water. Sens. Actuators B Chem. 2007, 123, 798–804. [Google Scholar] [CrossRef]
- Suvardhan, K.; Kumar, K.; Chiranjeevi, P. Extractive spectrofluorometric determination of quinalphos using fluorescein in environmental samples. Environ. Monit. Assess. 2005, 108, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Huang, J.R.; Wang, M.; Zhang, D.E.; Chen, J. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. J. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Silva, T.A.; Caetano, F.A.; Bergamini, M.F.; Marcolino, L.H., Jr.; Fatibello-Filho, O.; Janegitz, B.C. An overview of pesticide monitoring at environmental samples using carbon nanotubes-based electrochemical sensors. J. Carbon Res. 2017, 3, 8. [Google Scholar] [CrossRef]
- Deroco, P.B.; Lourencao, B.C.; Fatibello-Filho, O. The use of modified electrode with carbon black as sensor to the electrochemical studies and voltammetric determination of pesticide mesotrione. Microchem. J. 2017, 133, 188–194. [Google Scholar] [CrossRef]
- Baciu, A.; Ardelean, M.; Pop, A.; Pode, R.; Manea, F. Simultaneous voltammetric/amperometric determination of sulfide and nitrite in water at BDD electrode. Sensors 2015, 15, 14526–14538. [Google Scholar] [CrossRef] [PubMed]
- Tyszczuk-Rotko, K.; Bęczkowska, I.; Nosal-Wiercińska, A. Simple, selective and sensitive voltammetric method for the determination of herbicide (paraquat) using a bare boron-doped diamond electrode. Diam. Relat. Mater. 2014, 50, 86–90. [Google Scholar] [CrossRef]
- Pop, A.; Manea, F.; Radovan, C.; Dascalu, D.; Vaszilcsin, N.; Schoonman, J. Non-enzymatic electrochemical detection of glycerol on boron-doped diamond electrode. Analyst 2012, 137, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Švorc, Ľ.; Rievaj, M.; Bustin, D. Green electrochemical sensor for environmental monitoring of pesticides: Determination of atrazine in river waters using a boron-doped diamond electrode. Sens. Actuators B Chem. 2013, 181, 294–300. [Google Scholar] [CrossRef]
- Bandžuchová, L.; Švorc, Ľ.; Sochr, J.; Svítková, J.; Chýlková, J. Voltammetric method for sensitive determination of herbicide picloram in environmental and biological samples using boron-doped diamond film electrode. Electrochim. Acta 2013, 111, 242–249. [Google Scholar] [CrossRef]
- Bandžuchová, L.; Švorc, Ľ.; Vojs, M.; Marton, M.; Michniak, P.; Chýlková, J. Self-assembled sensor based on boron-doped diamond and its application in voltammetric analysis of picloram. Int. J. Environ. Anal. Chem. 2014, 94, 943–953. [Google Scholar] [CrossRef]
- Selva, T.M.G.; de Araujo, W.R.; Bacil, R.P.; Paixão, T.R.L.C. Study of Electrochemical Oxidation and Quantification of the Pesticide Pirimicarb Using a Boron-Doped Diamond Electrode. Electrochim. Acta 2017, 246, 588–596. [Google Scholar] [CrossRef]
- Rao, T.N.; Loo, B.H.; Sarada, B.V.; Terashima, C.; Fujishima, A. Electrochemical detection of carbamate pesticides at conductive diamond electrodes. Anal. Chem. 2002, 74, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, D.K.; Zadeh-Habchi, M.; Scorsone, E.; Bergonzo, P. Boron doped diamond/metal nanoparticle catalysts hybrid electrode array for the detection of pesticides in tap water. Proc. Eng. 2016, 168, 428–431. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of graphene in electrochemical sensing and biosensing. Trends Anal. Chem. 2016, 76, 1–14. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H.T. Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 2015, 84, 519–550. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modifed electrode. Talanta 2009, 79, 1406–1441. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.S.; Oh, K.H.; Park, J.K.; Yang, H. Rapid and SensitiveElectrochemical Detection of CarbarylBased on Enzyme Inhibition and Thiocholine OxidationMediated by a Ruthenium(III) Complex. Electroanalysis 2017, 29, 339–344. [Google Scholar] [CrossRef]
- De Oliveira, U.M.F.; Lichtig, J.; Masini, J.C. Evaluation of a nafion coated glassy carbon electrode for determination of paraquat by differential pulse voltammetry. J. Braz. Chem. Soc. 2004, 15, 735–741. [Google Scholar] [CrossRef]
- Saadati, N.; Abdullah, M.P.; Zakaria, Z.; Sany, S.B.T.; Rezayi, M.; Hassonizadeh, H. Limit of detection and limit of quantification development procedures for organochlorine pesticides analysis in water and sediment matrices. Chem. Cent. J. 2013, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Codognoto, L.; Tanimoto, S.T.; Pedrosa, V.A.; Suffredini, H.B.; Machado, S.A.S.; Avaca, L.A. Electroanalytical determination of carbaryl in natural waters on boron doped diamond electrode. Electroanalysis 2005, 18, 253–258. [Google Scholar] [CrossRef]
- Li, J.; Lei, W.; Xu, Y.; Zhang, Y.; Xia, M.; Wang, F. Fabrication of polypyrrole-grafted nitrogen-doped graphene and its application for electrochemical detection of paraquat. Electrochim. Acta 2015, 174, 464–471. [Google Scholar] [CrossRef]
- Baranowska, I.; Markowski, P.; Gerle, A.; Baranowki, J. Determination of selected drugs in human urine by differential pulse voltammetry technique. Bioelectrochemistry 2008, 73, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Laborda, E.; Molina, A.; Martinez-Ortiz, F.; Compton, R.G. Electrode modification using porous layers. Maximising the analytical response by choosing the most suitable voltammetry: Differential Pulse vs. Square Wave vs. Linear sweep voltammetry. Electrochim. Acta 2012, 73, 3–9. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, B.; Cui, L. Electrochemical analysis of carbaryl in fruit samples on graphene oxide-ionic liquid composite modified electrode. J. Food Compos. Anal. 2015, 40, 14–18. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Carreira, C.A.; Lee, H.J.; Silva, F.; Martins, A.; Pereira, C.M. Voltammetric determination of paraquat at DNA-gold nanoparticle composite electrodes. Electrochim. Acta 2010, 55, 7892–7896. [Google Scholar] [CrossRef]
- Garcia, L.L.C.; Figueiredo-Filho, L.C.S.; Oliveira, G.G.; Fatibello-Filho, O.; Banks, C.E. Square-wave voltammetric determination of paraquat using a glassy carbon electrode modified with multiwalled carbon nanotubes within a dihexadecylhydrogenphosphate (DHP) film. Sens. Actuators B Chem. 2013, 181, 306–311. [Google Scholar] [CrossRef]
- El Mhammedi, M.A.; Bakasse, M.; Bachirat, R.; Chtaini, A. Square wave voltammetry for analytical determination of paraquat at carbon paste electrode modified with fluoroapatite. Food Chem. 2008, 110, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.; Machado, S.A.S. Electrochemical detection of the herbicide paraquat in natural water and fruit juices using microelectrodes. Anal. Chim. Acta 2005, 546, 85–91. [Google Scholar] [CrossRef]






| Technique | Target Analyte | Parameters | Potential/V | Sensitivity (μA μM−1 cm−2) | LOD (μM) | LOQ (μM) | R2 |
|---|---|---|---|---|---|---|---|
| CV | CR | v = 0.05 Vs−1 | +1.47 | 1.85 | 0.14 | 0.46 | 0.966 |
| PQ | v = 0.05 Vs−1 | −0.78 | 46.12 | 0.01 | 0.04 | 0.981 | |
| DPV | CR | sp = 6 mV, MA = 800 mV | +0.74 | 30.5 | 0.07 | 0.23 | 0.985 |
| sp = 5 mV, MA = 50 mV | +1.42 | 1.39 | 0.16 | 0.55 | 0.999 | ||
| PQ | sp = 6 mV, MA = 800 mV | −0.80 | 30.8 | 0.04 | 0.13 | 0.993 | |
| sp = 5 mV, MA = 50 mV | −0.71 | 3.14 | 0.02 | 0.08 | 0.982 |
| Electrode | Analyte | Linear Range (μM) | LOD (μM) | Reference | |
|---|---|---|---|---|---|
| CR | PQ | ||||
| MWCNTs/Cobalt Phtalocyanine/GCE | x | - | 0.33–6.61 | 0.005 | [24] |
| Graphene oxide-ionic liquid/GCE | x | - | 0.10–12 | 0.02 | [32] |
| BDD | x | - | 1–30 | 0.041 | [28] |
| AuNPs/DNA/GE | - | x | 0–100 | 1.30 | [33] |
| MWCNTs-DHP/GCE | - | x | 0.20–1.70 | 0.026 | [34] |
| PPY-NGE/GCE | - | x | 0.05–2 | 0.041 | [29] |
| BDDGR | x | x | 1–6; 0.2–1.2 | 0.07; 0.01 | This work |
| Supporting Electrolyte | Target Analyte | Potential(V) | Sensitivity (μA μM−1 cm−2) | LOD (μM) | LOQ (μM) | R2 |
|---|---|---|---|---|---|---|
| Acetate Buffer | CR | +0.74 | 33.27 | 0.07 | 0.23 | 0.997 |
| PQ | −0.80 | 31.83 | 0.01 | 0.02 | 0.983 | |
| Natural Apple Juice | CR | +0.77 | 4.00 | 0.17 | 0.59 | 0.988 |
| PQ | −0.83 | 32.36 | 0.04 | 0.14 | 0.987 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, A.; Manea, F.; Flueras, A.; Schoonman, J. Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors 2017, 17, 2033. https://doi.org/10.3390/s17092033
Pop A, Manea F, Flueras A, Schoonman J. Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors. 2017; 17(9):2033. https://doi.org/10.3390/s17092033
Chicago/Turabian StylePop, Aniela, Florica Manea, Adriana Flueras, and Joop Schoonman. 2017. "Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode" Sensors 17, no. 9: 2033. https://doi.org/10.3390/s17092033
APA StylePop, A., Manea, F., Flueras, A., & Schoonman, J. (2017). Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors, 17(9), 2033. https://doi.org/10.3390/s17092033






