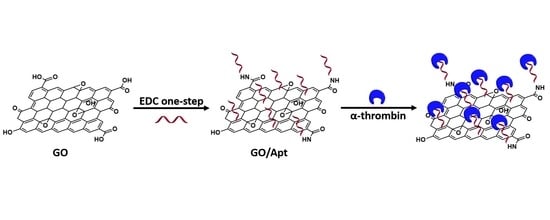
One-Step Facile Synthesis of Aptamer-Modified Graphene Oxide for Highly Specific Enrichment of Human A-Thrombin in Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Graphene Oxide
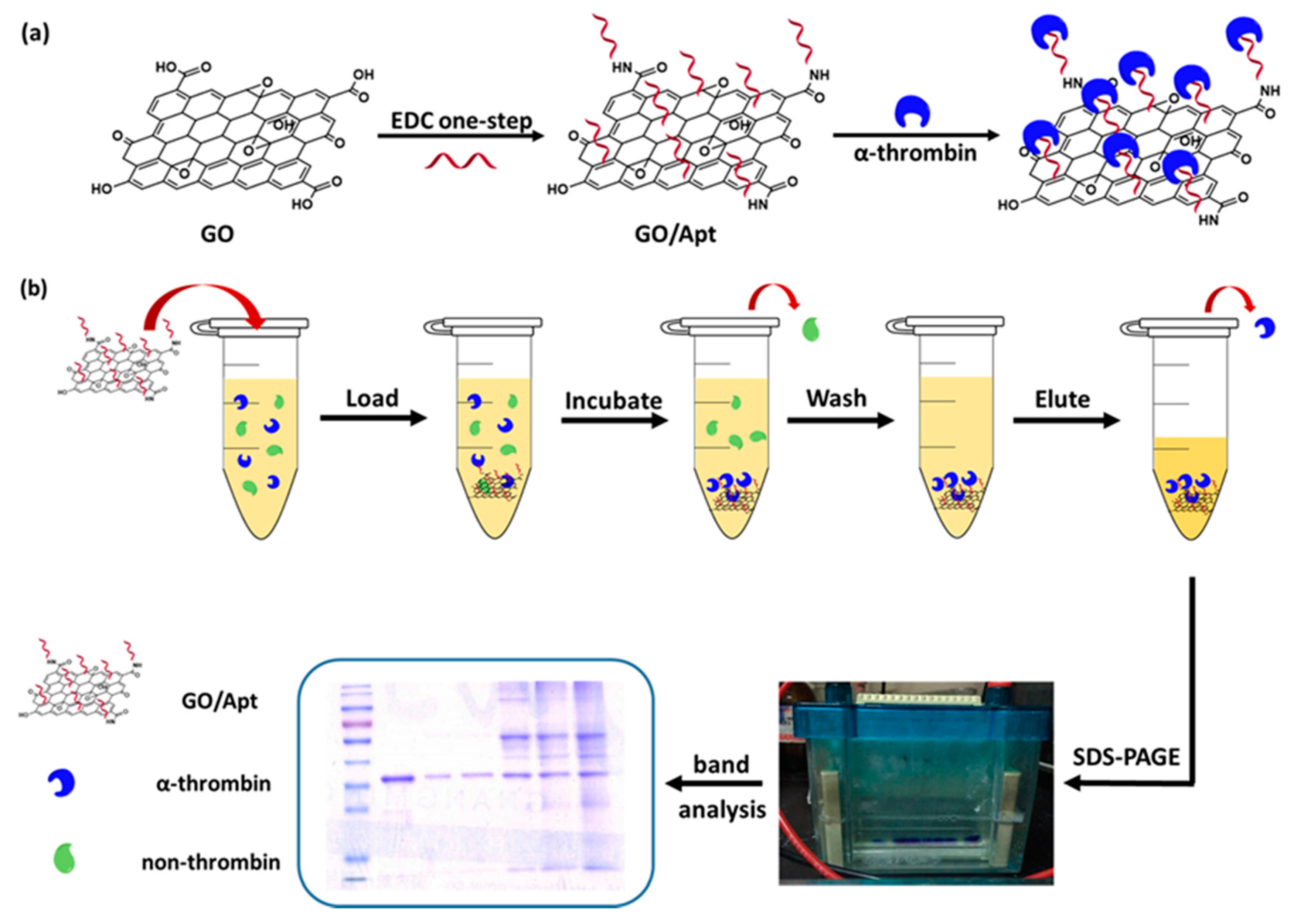
2.3. Preparation of GO/Apt Nanocomposites
2.4. Human α-Thrombin Enrichment with GO/Apt
2.5. Gel Electrophoresis
2.6. Characterization
3. Results and Discussion
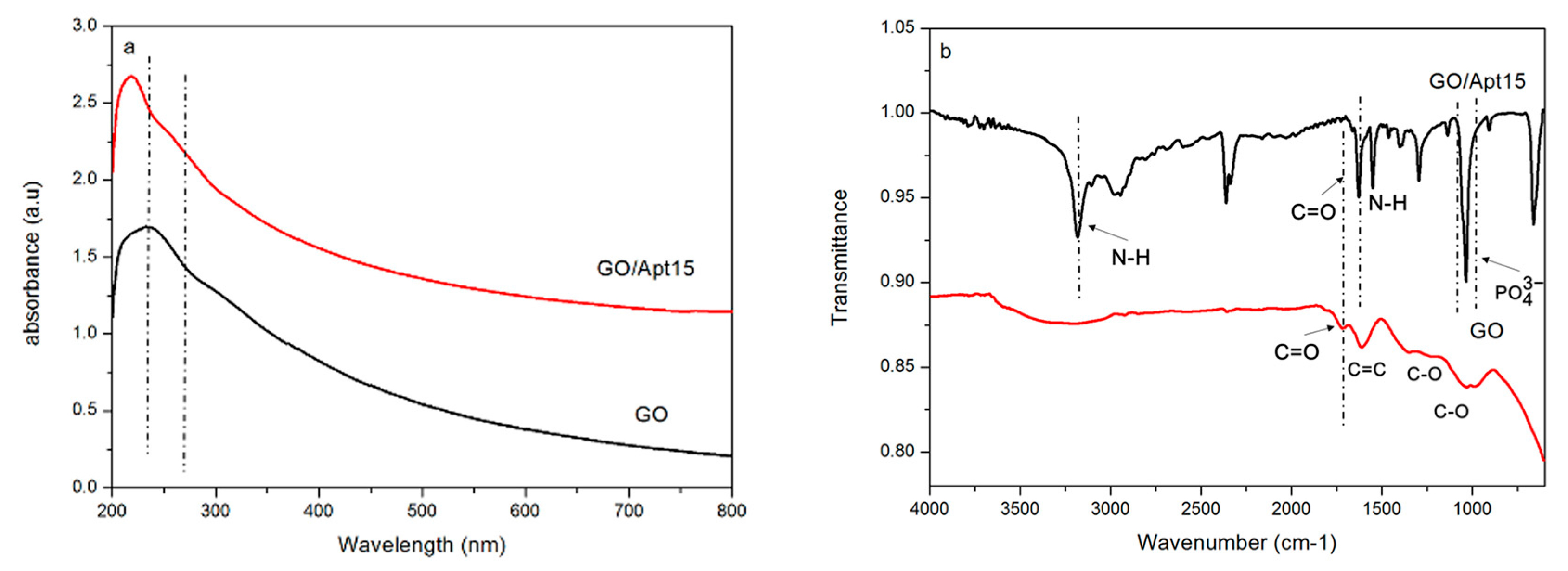
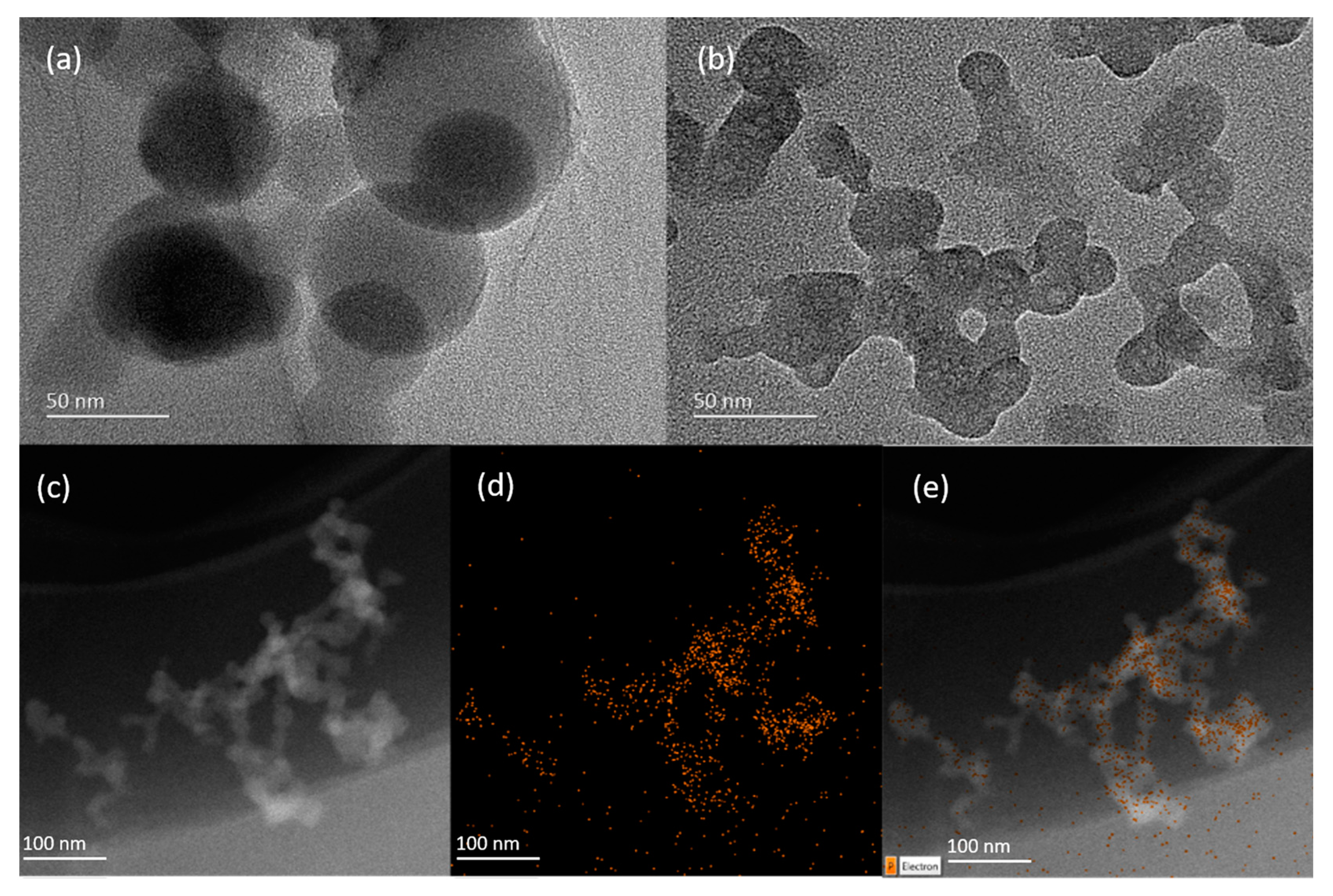
3.1. Synthesis and Characterization of GO/Apt Nanocomposites
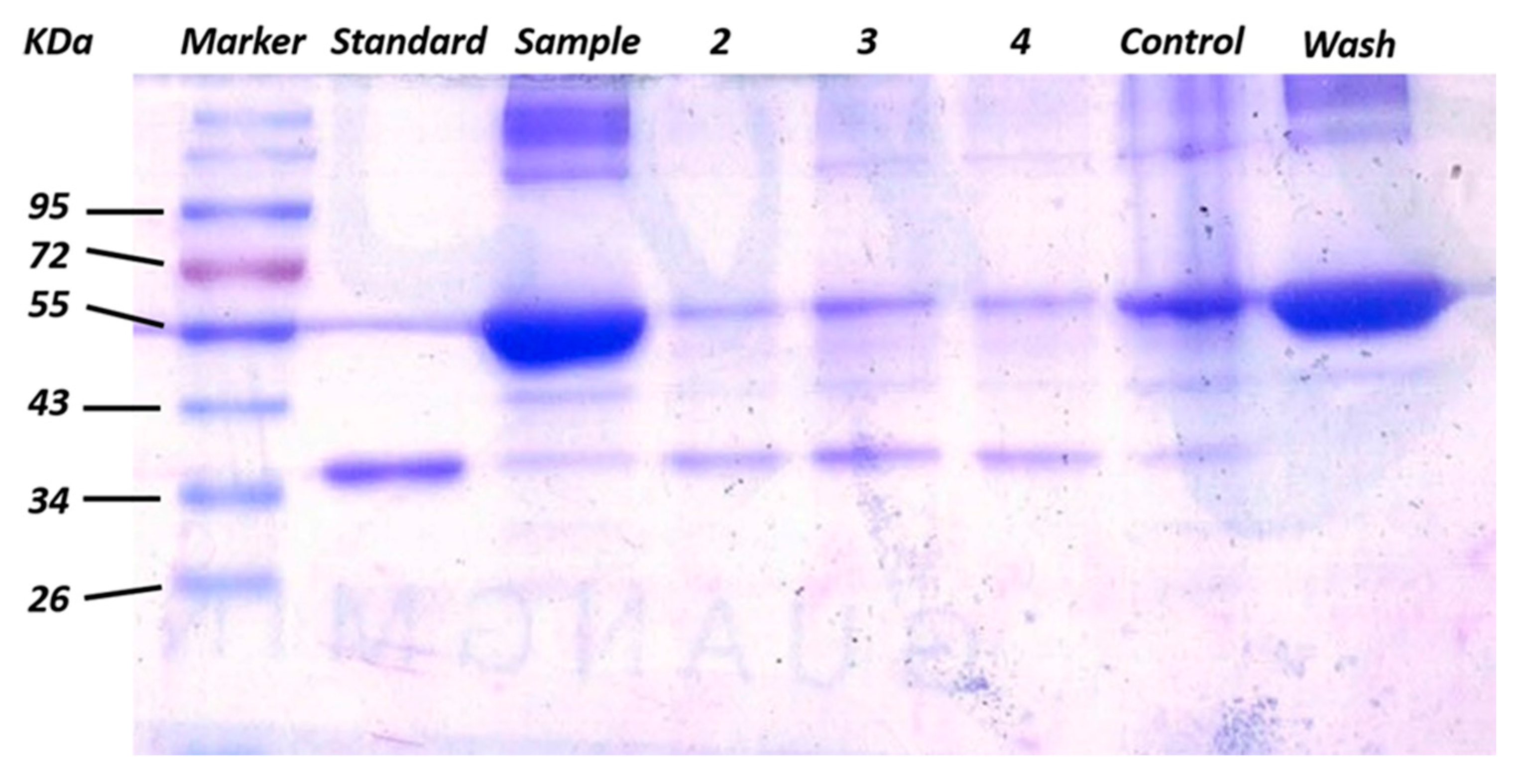
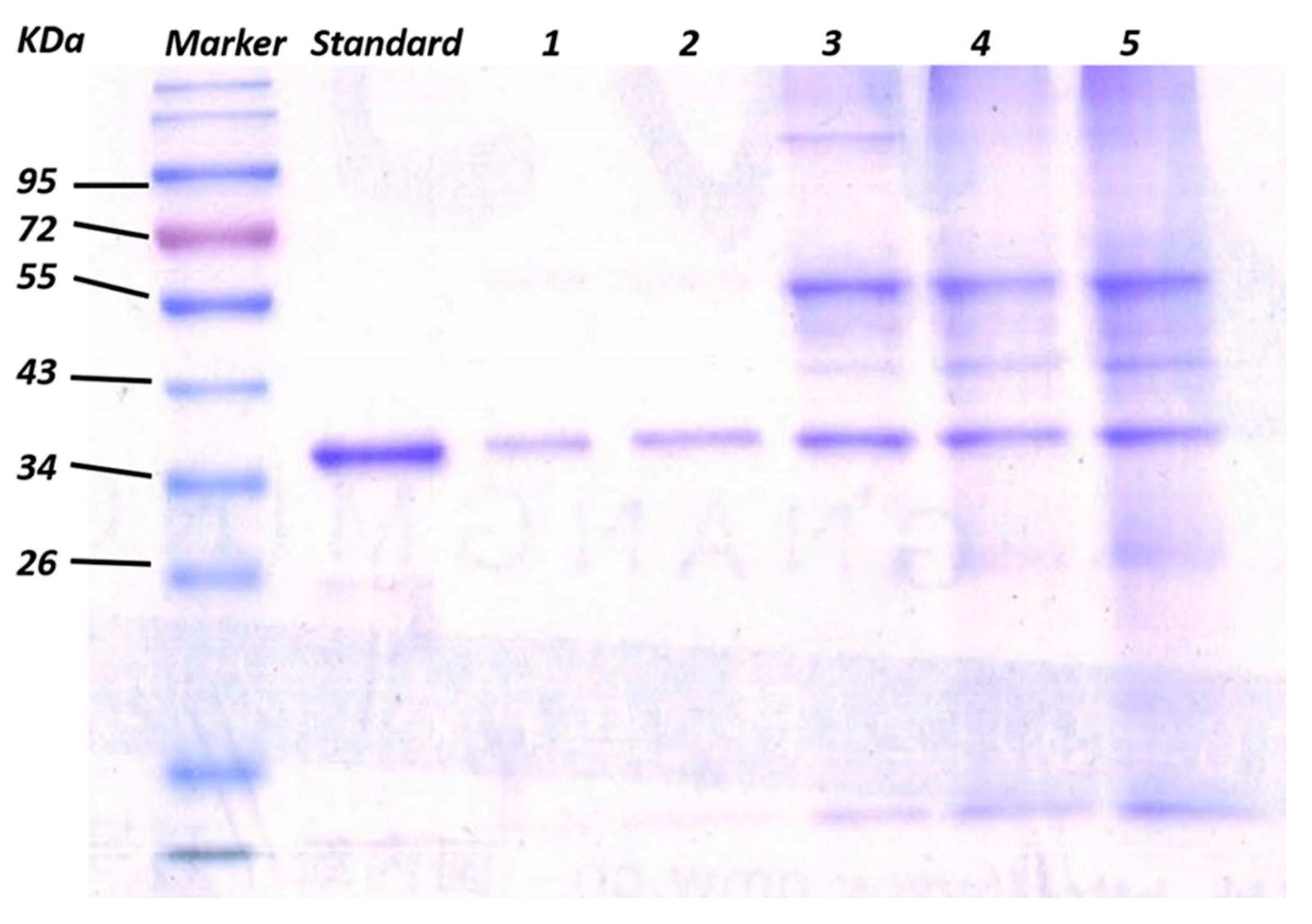
3.2. Enrichment and Recognition of Proteins by GO/Apt Nanocomposites
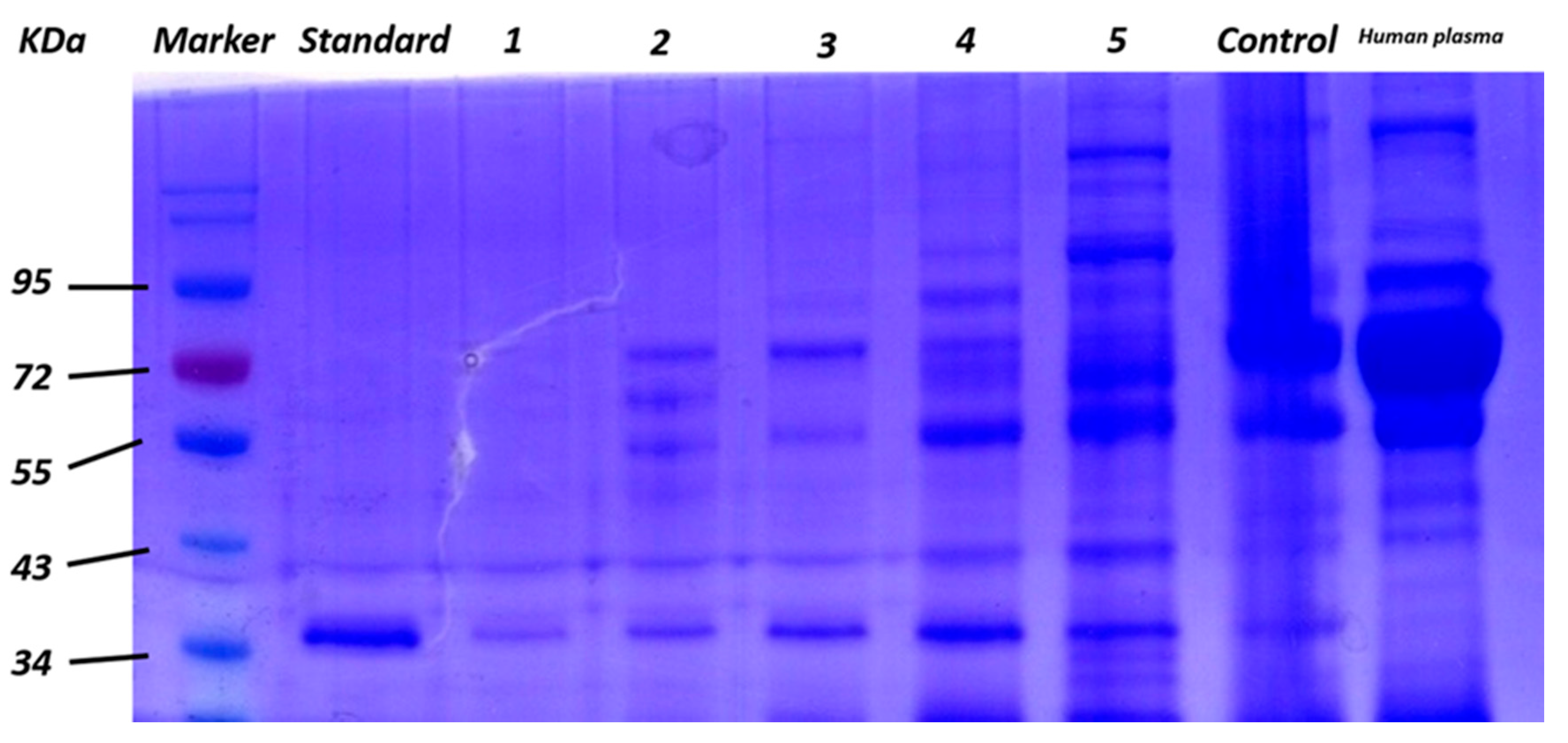
3.3. Application of GO/Apt in Human Plasma
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mu, B.; Zhang, J.; McNicholas, T.P.; Reuel, N.F.; Kruss, S.; Strano, M.S. Recent advances in molecular recognition based on nanoengineered platforms. Acc. Chem. Res. 2014, 47, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Tamburro, D.; Fredolini, C.; Espina, V.; Douglas, T.A.; Ranganathan, A.; Ilag, L.; Zhou, W.; Russo, P.; Espina, B.H.; Muto, G.; et al. Multifunctional core-shell nanoparticles: Discovery of previously invisible biomarkers. J. Am. Chem. Soc. 2011, 133, 19178–19188. [Google Scholar] [CrossRef] [PubMed]
- Huy, G.D.; Jin, N.; Yin, B.C.; Ye, B.C. A novel separation and enrichment method of 17beta-estradiol using aptamer-anchored microbeads. Bioprocess Biosyst. Eng. 2011, 34, 189–195. [Google Scholar] [PubMed]
- Ruigrok, V.J.; Levisson, M.; Eppink, M.H.; Smidt, H.; Van Der Oost, J. Alternative affinity tools: More attractive than antibodies? Biochem. J. 2011, 436, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.X.; Mann, M. A novel proteomic screen for peptide-protein interactions. J. Biol. Chem. 2004, 279, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.V.; Piro, B.; Reisberg, S.; Duc, H.T.; Pham, M.C. Antibodies directed to rna/DNA hybrids: An electrochemical immunosensor for micrornas detection using graphene-composite electrodes. Anal. Chem. 2013, 85, 8469–8474. [Google Scholar] [CrossRef] [PubMed]
- Iliuk, A.B.; Hu, L.; Tao, W.A. Aptamer in bioanalytical applications. Anal. Chem. 2011, 83, 4440–4452. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Nagasu, T.; Chait, B.T. Enrichment analysis of phosphorylated proteins as a tool for probing the phosphoproteome. Nat. Biotechnol. 2001, 19, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Guo, W.; Yu, H.; Zhao, J.; Pei, M. A novel electrochemical aptasensor based on mwcnts–bmimpf 6 and amino functionalized graphene nanocomposite films for determination of kanamycin. Anal. Methods 2015, 7, 5419–5427. [Google Scholar] [CrossRef]
- Xiong, Y.; Deng, C.; Zhang, X.; Yang, P. Designed synthesis of aptamer-immobilized magnetic mesoporous silica/au nanocomposites for highly selective enrichment and detection of insulin. ACS Appl. Mater. Interfaces 2015, 7, 8451–8456. [Google Scholar] [CrossRef] [PubMed]
- Misono, T.S.; Kumar, P.K. Selection of rna aptamers against human influenza virus hemagglutinin using surface plasmon resonance. Anal. Biochem. 2005, 342, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Cao, Z.; Meng, L.; Mallikaratchy, P.; Sefah, K.; Wang, H.; Li, Y.; Tan, W. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008, 7, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Sinha, S.S.; Garner, B.L.; Arany, I.; Corley, C.; Cobb, K.; Brown, E.; Ray, P.C. Influence of aptamer-enclosed silver nanocluster on the prevention of biofilm by bacillus thuringiensis. Nanosci. Nanotechnol. Lett. 2016, 8, 1054–1060. [Google Scholar] [CrossRef]
- Hong, K.L.; Sooter, L.J. Single-stranded DNA aptamers against pathogens and toxins: Identification and biosensing applications. BioMed Res. Int. 2015, 2015, 419318. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Gariano, N.A.; Spivak, D.A. Macromolecular amplification of binding response in superaptamer hydrogels. J. Am. Chem. Soc. 2013, 135, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, J.; Liu, L.; Li, C.; Ju, H. Self-assembled DNA hydrogel as switchable material for aptamer-based fluorescent detection of protein. Anal. Chem. 2013, 85, 11077–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hong, Y.; Xiao, Z.; Zhou, Y.; Jiang, Y.; Huang, M.; Xu, X.; Zhou, G. Colorimetric determination of salmonella typhimurium based on aptamer recognition. Anal. Methods 2016, 8, 6560–6565. [Google Scholar] [CrossRef]
- Niu, X.; Huang, L.; Zhao, J.; Yin, M.; Luo, D.; Yang, Y. An ultrasensitive aptamer biosensor for the detection of codeine based on a au nanoparticle/polyamidoamine dendrimer-modified screen-printed carbon electrode. Anal. Methods 2016, 8, 1091–1095. [Google Scholar] [CrossRef]
- Deng, N.; Liang, Z.; Liang, Y.; Sui, Z.; Zhang, L.; Wu, Q.; Yang, K.; Zhang, L.; Zhang, Y. Aptamer modified organic-inorganic hybrid silica monolithic capillary columns for highly selective recognition of thrombin. Anal. Chem. 2012, 84, 10186–10190. [Google Scholar] [CrossRef] [PubMed]
- Madru, B.; Chapuis-Hugon, F.; Peyrin, E.; Pichon, V. Determination of cocaine in human plasma by selective solid-phase extraction using an aptamer-based sorbent. Anal. Chem. 2009, 81, 7081–7086. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, M.E.; Khayamian, T.; Hashemian, Z. Aptamer-conjugated magnetic nanoparticles for extraction of adenosine from urine followed by electrospray ion mobility spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Dick, L.W.; McGown, L.B. Aptamer-enhanced laser desorption/ionization for affinity mass spectrometry. Anal. Chem. 2004, 76, 3037–3041. [Google Scholar] [CrossRef] [PubMed]
- Yasun, E.; Gulbakan, B.; Ocsoy, I.; Yuan, Q.; Shukoor, M.I.; Li, C.; Tan, W. Enrichment and detection of rare proteins with aptamer-conjugated gold nanorods. Anal. Chem. 2012, 84, 6008–6015. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Deng, C.; Zhang, X. Development of aptamer-conjugated magnetic graphene/gold nanoparticle hybrid nanocomposites for specific enrichment and rapid analysis of thrombin by maldi-tof ms. Talanta 2014, 129, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Brothier, F.; Combes, A. Aptamer-based-sorbents for sample treatment—A review. Anal. Bioanal. Chem. 2015, 407, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Danquah, M.K.; Yon, J.L.; Sidhu, A.; Ongkudon, C.M. A review on immobilised aptamers for high throughput biomolecular detection and screening. Anal. Chim. Acta 2015, 888, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Guo, L.; Qin, Q.; Zheng, X.; Ruan, G.; Li, J.; Li, G. Recent advances in aptamer-functionalized materials in sample preparation. TrAC Trends Anal. Chem. 2015, 67, 134–146. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Yan, D.; Lu, H. Deposition of Fe–Ni nanoparticles on polyethyleneimine-decorated graphene oxide and application in catalytic dehydrogenation of ammonia borane. J. Mater. Chem. 2012, 22, 13506–13516. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, K.; Zhao, Q.; Wu, Q.; Liang, Z.; Zhang, L.; Peng, X.; Zhang, Y. Hydrophilic immobilized trypsin reactor with magnetic graphene oxide as support for high efficient proteome digestion. J. Chromatogr. A 2012, 1254, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Huang, Y.; Tian, J.; Hu, K.; Pan, L.; Zhao, S. A novel exonuclease iii-aided amplification assay based on a graphene platform for sensitive detection of adenosine triphosphate. Anal. Methods 2015, 7, 3708–3713. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Bai, H.; Pan, Y.; Tong, W.; Qin, P.; Yan, H.; Deng, S.; Zhong, R.; Qin, W.; Qian, X. A graphene oxide-based immobilized pngase f reagent for highly efficient n-glycan release and maldi-tof ms profiling. Anal. Methods 2014, 6, 2518–2525. [Google Scholar] [CrossRef]
- Park, J.W.; Tatavarty, R.; Kim, D.W.; Jung, H.T.; Gu, M.B. Immobilization-free screening of aptamers assisted by graphene oxide. Chem. Commun. 2012, 48, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, S.; Deng, C.; Zhang, X. Highly sensitive thrombin detection by matrix assisted laser desorption ionization-time of flight mass spectrometry with aptamer functionalized core–shell fe 3 o 4@ c@ au magnetic microspheres. Talanta 2012, 88, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Jiang, B.; Chen, Y.; Liang, Z.; Zhang, L.; Liang, Y.; Yang, K.; Zhang, Y. Aptamer-conjugated gold functionalized graphene oxide nanocomposites for human alpha-thrombin specific recognition. J. Chromatogr. A 2016, 1427, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Obubuafo, A.; McCarley, R.L.; Soper, S.A.; Spivak, D.A. Effect of linker structure on surface density of aptamer monolayers and their corresponding protein binding efficiency. Anal. Chem. 2008, 80, 9630–9634. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Wang, C.; Zhang, J.; Li, X.-F.; Le, X.C. Competitive protection of aptamer-functionalized gold nanoparticles by controlling the DNA assembly. Anal. Chem. 2011, 83, 6464–6467. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Tan, S.; Liang, Q.; Ding, M. One-Step Facile Synthesis of Aptamer-Modified Graphene Oxide for Highly Specific Enrichment of Human A-Thrombin in Plasma. Sensors 2017, 17, 1986. https://doi.org/10.3390/s17091986
Xu Y, Tan S, Liang Q, Ding M. One-Step Facile Synthesis of Aptamer-Modified Graphene Oxide for Highly Specific Enrichment of Human A-Thrombin in Plasma. Sensors. 2017; 17(9):1986. https://doi.org/10.3390/s17091986
Chicago/Turabian StyleXu, Yuan, Siyuan Tan, Qionglin Liang, and Mingyu Ding. 2017. "One-Step Facile Synthesis of Aptamer-Modified Graphene Oxide for Highly Specific Enrichment of Human A-Thrombin in Plasma" Sensors 17, no. 9: 1986. https://doi.org/10.3390/s17091986
APA StyleXu, Y., Tan, S., Liang, Q., & Ding, M. (2017). One-Step Facile Synthesis of Aptamer-Modified Graphene Oxide for Highly Specific Enrichment of Human A-Thrombin in Plasma. Sensors, 17(9), 1986. https://doi.org/10.3390/s17091986