Single Exhale Biomarker Breathalyzer
Abstract
:1. Introduction
2. Experimental Method
Calibration Procedure
3. Experimental Results
3.1. Readout Front-End
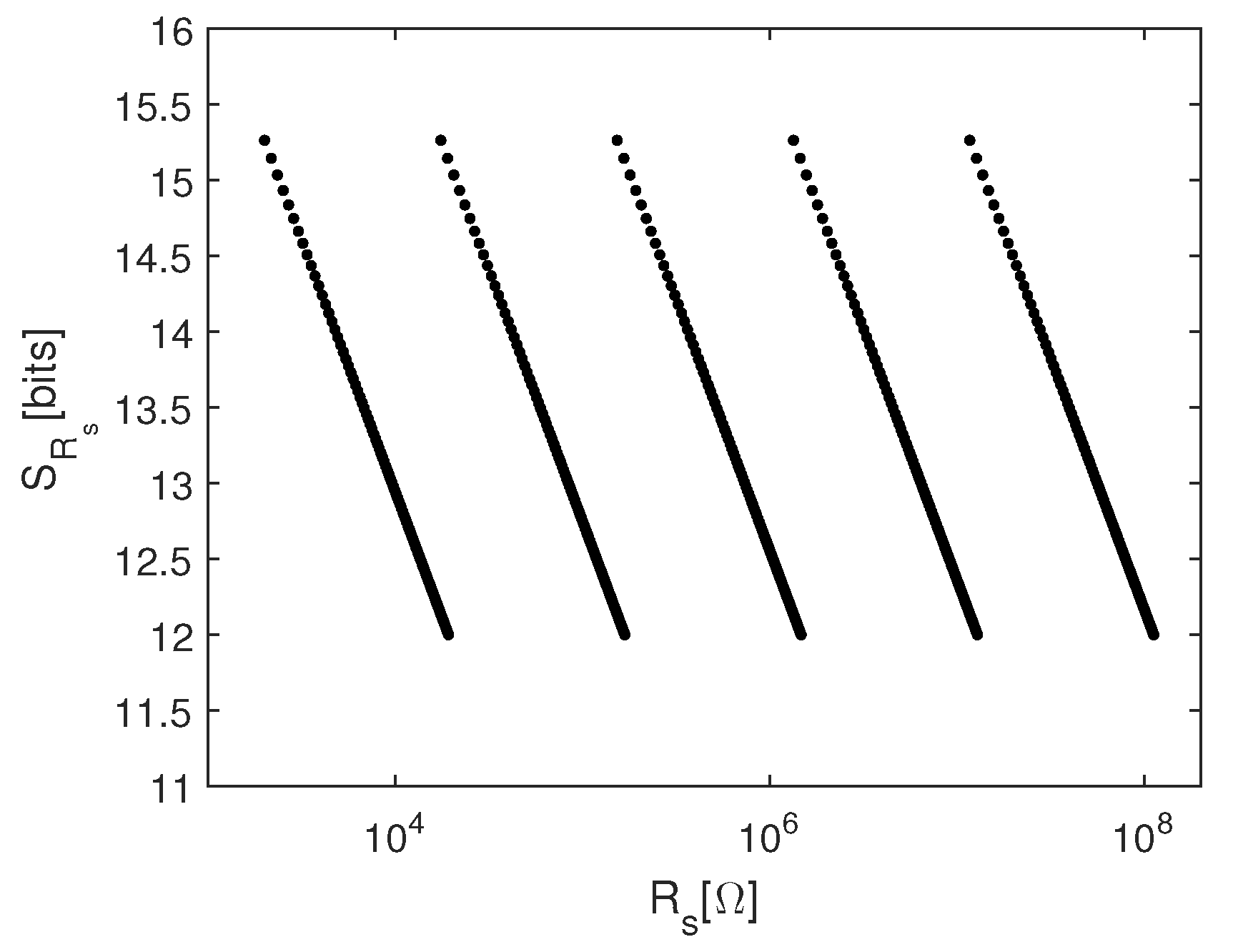
3.1.1. Calibration
3.1.2. Noise
3.2. Characterization Experiments Using Data Acquisition System
3.2.1. Reliability of the Sensor Response
3.2.2. Characterization of Sensor Response Dynamics
3.2.3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manolis, A. The diagnostic potential of breath analysis. Clin. Chem. 1983, 29, 5–15. [Google Scholar] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Gouma, P.; Prasad, A.; Stanacevic, S. A selective nanosensor device for exhaled breath analysis. J. Breath Res. 2011, 5, 037110. [Google Scholar] [CrossRef] [PubMed]
- Risby, T.; Solga, S. Current status of clinical breath analysis. Appl. Phys. B 2006, 85, 421–426. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef]
- NIOX. Available online: http://www.niox.com (accessed on 9 January 2019).
- Guo, D.; Zhang, D.; Li, N.; Zhang, L.; Yang, J. A Novel Breath Analysis System Based on Electronic Olfaction. IEEE Trans. Biomed. Eng. 2010, 57, 2753–2763. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, D.; Wu, D.; Wei, H.; Lu, G. Design of a Breath Analysis System for Diabetes Screening and Blood Glucose Level Prediction. IEEE Trans. Biomed. Eng. 2014, 61, 2787–2795. [Google Scholar] [CrossRef]
- Huang, C.H.; Zeng, C.; Wang, Y.C.; Peng, H.Y.; Lin, C.S.; Chang, C.J.; Yang, H.Y. A Study of Diagnostic Accuracy Using a Chemical Sensor Array and a Machine Learning Technique to Detect Lung Cancer. Sensors 2018, 18, 2845. [Google Scholar] [CrossRef]
- Shurmer, H.V.; Gardner, J.W. Odour discrimination with an electronic nose. Sens. Actuators B Chem. 1992, 8, 1–11. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic Nose: Current Status and Future Trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [Green Version]
- Falasconi, M.; Pardo, M.; Sberveglieri, G.; Riccò, I.; Bresciani, A. The novel EOS835 electronic nose and data analysis for evaluating coffee ripening. Sens. Actuators B Chem. 2005, 110, 73–80. [Google Scholar] [CrossRef]
- Artursson, T.; Eklöv, T.; Lundström, I.; Mårtensson, P.; Sjöström, M.; Holmberg, M. Drift correction for gas sensors using multivariate methods. J. Chemom. 2000, 14, 711–723. [Google Scholar] [CrossRef]
- Vergara, A.; Vembu, S.; Ayhan, T.; Ryan, M.A.; Homer, M.L.; Huerta, R. Chemical gas sensor drift compensation using classifier ensembles. Sens. Actuators B Chem. 2012, 166, 320–329. [Google Scholar] [CrossRef]
- Ziyatdinov, A.; Marco, S.; Chaudry, A.; Persaud, K.; Caminal, P.; Perera, A. Drift compensation of gas sensor array data by common principal component analysis. Sens. Actuators B Chem. 2010, 146, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Gouma, P.I.; Kalyanasundaram, K. A selective nanosensing probe for nitric oxide. Appl. Phys. Lett. 2008, 93, 244102. [Google Scholar] [CrossRef]
- Gouma, P.; Sood, S.; Stanacevic, M.; Simon, S. Selective Chemosensing and Diagnostic Breathanalyzer. Procedia Eng. 2014, 87, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.W.; Xu, J.C.; Biaggi-Labiosa, A.M.; Laskowski, D.; Dutta, P.K.; Mondal, S.P.; Ward, B.J.; Makel, D.B.; Liu, C.C.; Chang, C.W.; et al. Smart sensor systems for human health breath monitoring applications. J. Breath Res. 2011, 5, 037111. [Google Scholar] [CrossRef]
- Mondal, S.P.; Dutta, P.K.; Hunter, G.; Ward, B.; Laskowski, D.; Dweik, R. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sens. Actuators B Chem. 2011, 158, 292–298. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Sood, S.; Gouma, P. Breath Biomarker Detection by Chemical Sensors. In Semiconductor-Based Sensors; Ren, F., Pearton, S.J., Eds.; World Scientific: Singapore, 2016; pp. 355–393. [Google Scholar]
- Wang, L.; Gouma, P. Selective Microstructure Synthesis and Sensing Dependencies; Springer: New York, NY, USA, 2013; Chapter 5; pp. 167–188. [Google Scholar] [CrossRef]
- Gouma, P.I.; Prasad, A.K.; Iyer, K.K. Selective nanoprobes for ‘signalling gases’. Nanotechnology 2006, 17, S48. [Google Scholar] [CrossRef]
- Grassi, M.; Malcovati, P.; Baschirotto, A. A 141-dB Dynamic Range CMOS Gas-Sensor Interface Circuit Without Calibration with 16-Bit Digital Output Word. IEEE J. Solid-State Circuits 2007, 42, 1543–1554. [Google Scholar] [CrossRef]
- Barrettino, D.; Graf, M.; Taschini, S.; Hafizovic, S.; Hagleitner, C.; Hierlemann, A. CMOS Monolithic Metal-Oxide Gas Sensor Microsystems. IEEE Sens. J. 2006, 6, 276–286. [Google Scholar] [CrossRef]
- Grassi, M.; Malcovati, P.; Baschirotto, A. A 160-dB Equivalent Dynamic Range Auto-scaling Interface for Resistive Gas Sensor Arrays. IEEE J. Solid-State Circuits 2007, 42, 518–528. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Mason, A. Highly Adaptive Transducer Interface Circuit for Multiparameter Microsystems. IEEE Trans. Circuits Syst. 2007, 54, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Gouma, P.; Stanaćević, M. A Low-Power Wide-Dynamic-Range Readout IC for Breath Analyzer System. In Proceedings of the 2013 IEEE International Symposium on Circuits and Systems (ISCAS2013), Beijing, China, 19–23 May 2013. [Google Scholar]
- Gatty, H.K.; Leijonmarck, S.; Antelius, M.; Stemme, G.; Roxhed, N. An amperometric nitric oxide sensor with fast response and ppb-level concentration detection relevant to asthma monitoring. Sens. Actuators B Chem. 2015, 209, 639–644. [Google Scholar] [CrossRef]
- Zheng, Z.; Ren, H.; VonWald, I.; Meyerhoff, M.E. Highly sensitive amperometric Pt–Nafion gas phase nitric oxide sensor: Performance and application in characterizing nitric oxide-releasing biomaterials. Anal. Chim. Acta 2015, 887, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, Y.; Lin, Y.; Jodhani, G.; Stanaćević, M.; Gouma, P.-I. Single Exhale Biomarker Breathalyzer. Sensors 2019, 19, 270. https://doi.org/10.3390/s19020270
Karimi Y, Lin Y, Jodhani G, Stanaćević M, Gouma P-I. Single Exhale Biomarker Breathalyzer. Sensors. 2019; 19(2):270. https://doi.org/10.3390/s19020270
Chicago/Turabian StyleKarimi, Yasha, Yingkan Lin, Gagan Jodhani, Milutin Stanaćević, and Pelagia-Irene Gouma. 2019. "Single Exhale Biomarker Breathalyzer" Sensors 19, no. 2: 270. https://doi.org/10.3390/s19020270



