Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Solutions
2.2. Electrochemical Measurements
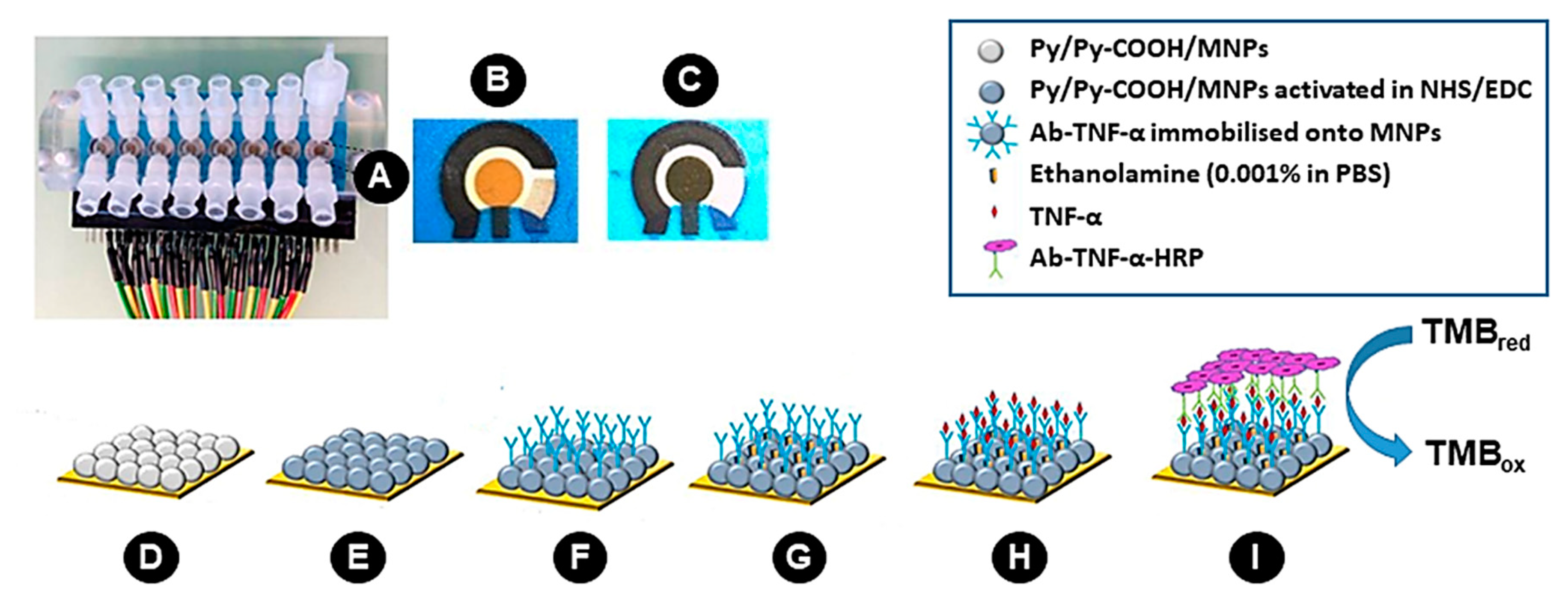
2.3. Coating of Magnetic Microparticles with Poly (pyrrole-co-pyrrole-2-carboxylic Acid)
2.4. Preparation of 2D-SPEAu
2.5. Preparation of 3D-SPEAu
2.6. Electrode Surface and Microparticles Characterization
2.7. Standard Solutions and Sample Analysis
3. Results and Discussion
3.1. Electrode Surface and Microparticles Characterization
3.2. Voltammetric Studies
3.3. Detection of TNF-α in Standard Solution and Interference Study
3.4. Detection TNF-α in Artificial Saliva with the 3D-SPEAu Immunosensor
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Umapathy, D.; Krishnamoorthy, E.; Mariappanadar, V.; Viswanathan, V.; Ramkumar, K.M. Increased levels of circulating (TNF-α) is associated with (-308G/A) promoter polymorphism of TNF-α gene in Diabetic Nephropathy. Int. J. Biol. Macromol. 2018, 107, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Fantuzzi, F.; Del Giglio, M.; Gisondi, P.; Girolomoni, G. Targeting tumor necrosis factor alpha in psoriasis and psoriatic arthritis. Expert. Opin. Ther. Targets 2008, 12, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Pache, I.; Rogler, G.; Felley, C. TNF-alpha blockers in inflammatory bowel diseases: Practical consensus recommendations and a user’s guide. Swiss. Med. Wkly. 2009, 139, 278–287. [Google Scholar] [PubMed]
- Bozkurt, B. Activation of cytokines as a mechanism of disease progression in heart failure. Ann. Rheum. Dis. 2000, 59, i90–i93. [Google Scholar] [CrossRef]
- Yndestad, A.; Damås, J.K.; Oie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Systemic inflammation in heart failure—The whys and wherefores. Heart Fail. Rev. 2006, 11, 83–92. [Google Scholar] [CrossRef]
- Chen, D.; Assad-Kottner, C.; Orrego, C.; Torre-Amione, G. Cytokines and acute heart failure. Crit. Care Med. 2008, 36, S9–S16. [Google Scholar] [CrossRef]
- Guha, K.; McDonagh, T. Heart failure epidemiology: European perspective. Curr. Cardiol. Rev. 2013, 9, 123–127. [Google Scholar] [CrossRef]
- Hofman, L.F. Human saliva as a diagnostic specimen. J. Nutr. 2001, 131, 1621s–1625s. [Google Scholar] [CrossRef] [PubMed]
- Tripoliti, E.; Papadopoulos, T.; Karanasiou, G.; Kalatzis, F.; Goletsis, Y.; Ghimenti, S.; Lomonaco, T.; Bellagambi, F.; Trivella, M.G.; Fuoco, R.; et al. A computational approach for the estimation of heart failure patients’ status using saliva biomarkers. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3648–3651. [Google Scholar] [CrossRef] [PubMed]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of salivary α-amylase and cortisol in psoriatic subjects undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Pezelj-Ribaric, S.; Prso, I.B.; Abram, M.; Glazar, I.; Brumini, G.; Simunovic-Soskic, M. Salivary levels of tumor necrosis factor-alpha in oral lichen planus. Mediat. Inflamm. 2004, 13, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Yousefimanesh, H.; Maryam, R.; Mahmoud, J.; Mehri, G.B.; Mohsen, T. Evaluation of salivary tumor necrosis factor-alpha in patients with the chronic periodontitis: A case-control study. J. Indian Soc. Periodontol. 2013, 17, 737–740. [Google Scholar] [CrossRef]
- Gumus, P.; Nizam, N.; Lappin, D.F.; Buduneli, N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-α in patients with periodontitis. J. Periodontol. 2014, 85, 270–280. [Google Scholar] [CrossRef]
- Mire-Sluis, A.R.; Gaines-Das, R.; Thorpe, R. Immunoassays for detecting cytokines: What are they really measuring? J. Immunol. Methods 1995, 186, 157–160. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Ivashkiv, L.B. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J. Immunol. 2000, 165, 5227–5237. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Baraket, A.; Kabbaj, D.; Zine, N.; Errachid, A.; Fessi, H.; Kunita, M.H.; Elaissari, A. Submicron magnetic core conducting polypyrrole polymer shell: Preparation and characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 688–694. [Google Scholar] [CrossRef]
- Hassani, N.E.A.E.; Baraket, A.; Tenório-Neto, E.T.; Lee, M.; Salvador, J.P.; Marco, M.P.; Bausells, J.; Bari, N.E.; Bouchikhi, B.; Elaissari, A.; et al. Novel strategy for sulfapyridine detection using a fully integrated electrochemical Bio-MEMS: Application to honey analysis. Biosens. Bioelectron. 2017, 93, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.; Baraket, A.; Bellagambi, F.G.; Karanasiou, G.S.; Ben Ali, M.; Fotiadis, D.I.; Bausells, J.; Zine, N.; Sigaud, M.; Errachid, A. A novel chronoamperometric immunosensor for rapid detection of TNF-α in human saliva. Sens. Actuators B Chem. 2018, 266, 477–484. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Baraket, A.; Longo, A.; Vatteroni, M.; Zine, N.; Bausells, J.; Fuoco, R.; Di Francesco, F.; Karanasiou, G.S.; Fotiadis, D.I.; et al. Electrochemical biosensor platform for TNF-α cytokines detection in both artificial and human saliva: Heart failure. Sens. Actuators. B Chem. 2017, 251, 1026–1033. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Yaakoubi, N.; Trivella, M.G.; Zabala, M.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. Diazonium modified gold microelectrodes onto polyimide substrates for impedimetric cytokine detection with an integrated Ag/AgCl reference electrode. Sens. Actuators B Chem. 2013, 189, 165–172. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A highly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef]




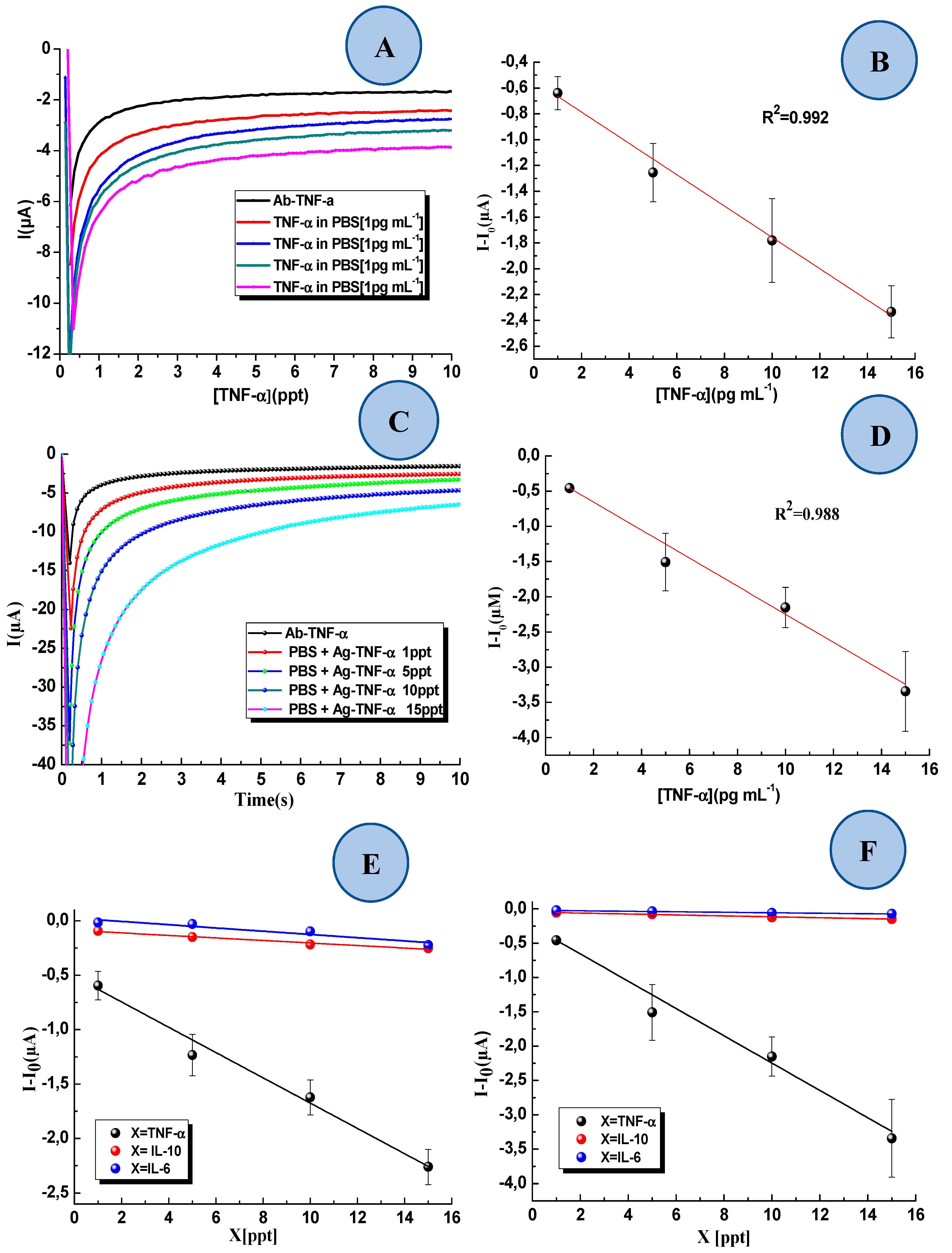

| Standard Solution Containing | Calibration Curve (R2) | |
|---|---|---|
| 2D-SPEAu | 3D-SPEAu | |
| TNF-α | y = –0.1161x – 0.5130 (0.984) | y = –0.1990x – 0.2575 (0.988) |
| IL-6 | y = –0.0149x – 0.0242 (0.873) | y = –0.0036x – 0.0205 (0.971) |
| IL-10 | y = –0.0161x – 0.0876 (0.967) | y = –0.0068x – 0.0476 (0.974) |
| Immunosensor with | R2 | LoD [pg·mL−1] | CV% | Sensitivity (µA pg·mL−1) |
|---|---|---|---|---|
| 2D-SPEAu | 0.8423 | 0.3 | 22 | 0.116 |
| 3D-SPEAu | 0.9812 | 0.3 | 8 | 0.199 |
| Technique | Linear Range | LoD | Reference |
|---|---|---|---|
| Impedance spectroscopy | 1–100 pg·mL−1 | 1 pg·mL−1 | [23] |
| Impedance spectroscopy | 0.01–2 pg·mL−1 | 3.7 fg·mL−1 | [27] |
| Amperometry | 1–200 pg·mL−1 | 0.85 pg·mL−1 | [28] |
| Amperometry | 1–30 pg·mL−1 | 1 pg·mL−1 | [22] |
| Amperometry | 1–15 pg·mL−1 | 0.3 pg·mL−1 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barhoumi, L.; Bellagambi, F.G.; Vivaldi, F.M.; Baraket, A.; Clément, Y.; Zine, N.; Ben Ali, M.; Elaissari, A.; Errachid, A. Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode. Sensors 2019, 19, 692. https://doi.org/10.3390/s19030692
Barhoumi L, Bellagambi FG, Vivaldi FM, Baraket A, Clément Y, Zine N, Ben Ali M, Elaissari A, Errachid A. Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode. Sensors. 2019; 19(3):692. https://doi.org/10.3390/s19030692
Chicago/Turabian StyleBarhoumi, Lassaad, Francesca G. Bellagambi, Federico M. Vivaldi, Abdoullatif Baraket, Yohann Clément, Nadia Zine, Mounir Ben Ali, Abdelhamid Elaissari, and Abdelhamid Errachid. 2019. "Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode" Sensors 19, no. 3: 692. https://doi.org/10.3390/s19030692





