A Cost-Effective and Portable Optical Sensor System to Estimate Leaf Nitrogen and Water Contents in Crops
Abstract
:1. Introduction
2. Materials and Methods
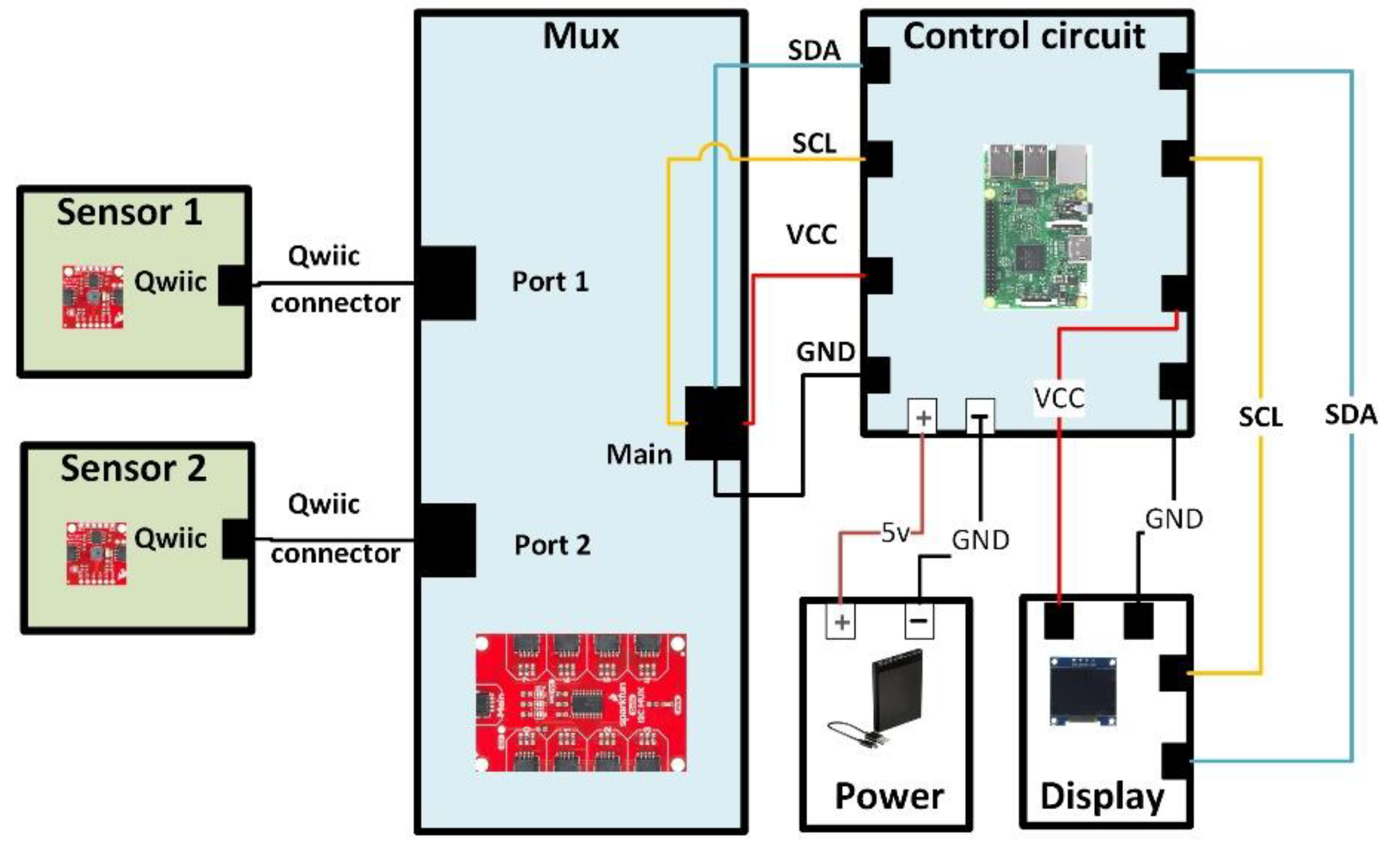
2.1. Designed Hardware Sensing System
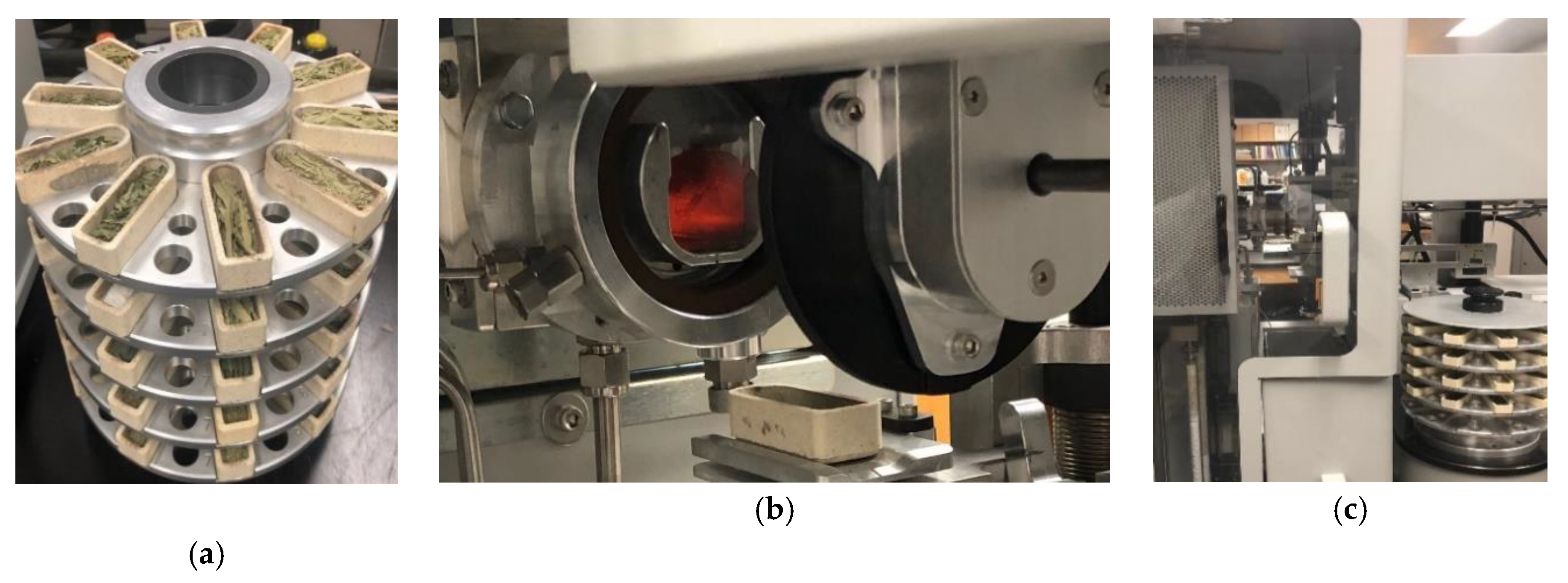
2.2. Greenhouse Experimental Set-Up
2.3. Spectral Data Collection and Ground Truth Measurement
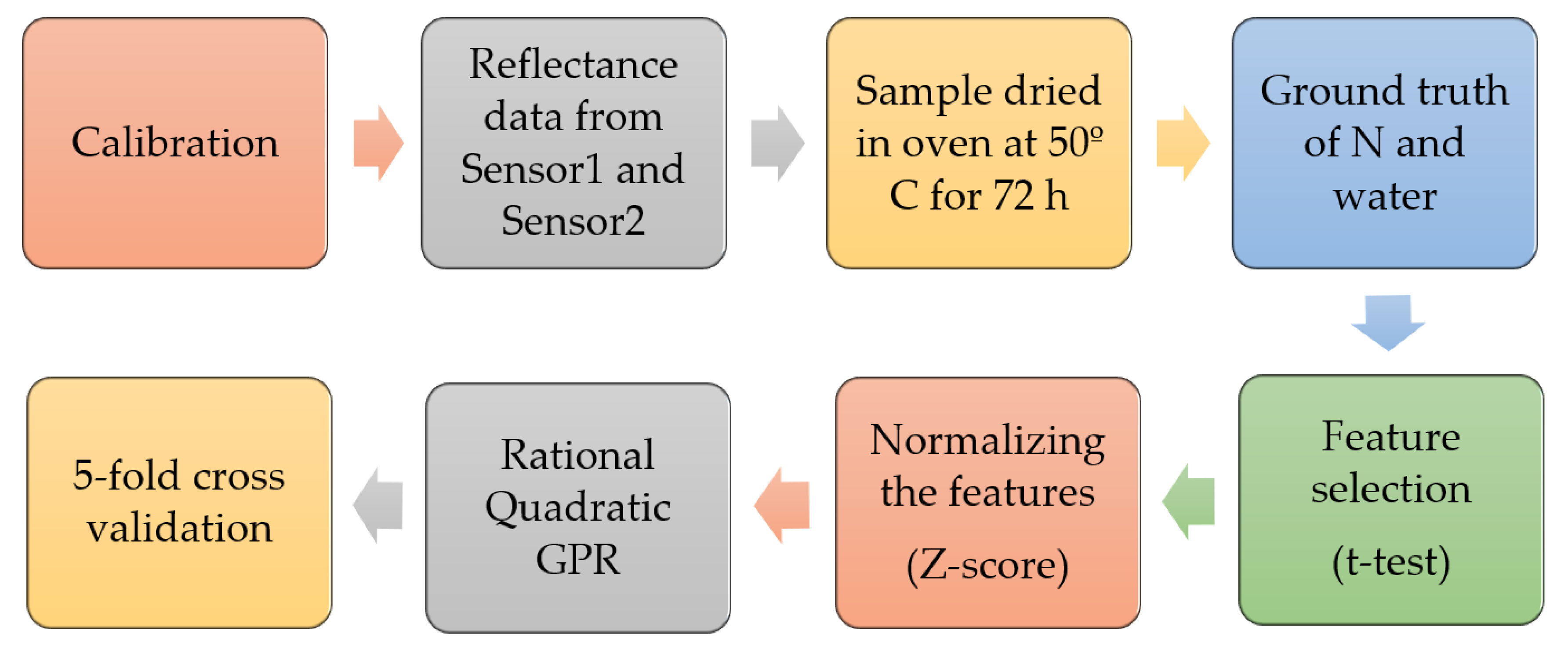
2.4. Data Preprocessing and Modeling
2.5. Validation Metrics
3. Results
3.1. Nitrogen Concentration Estimation
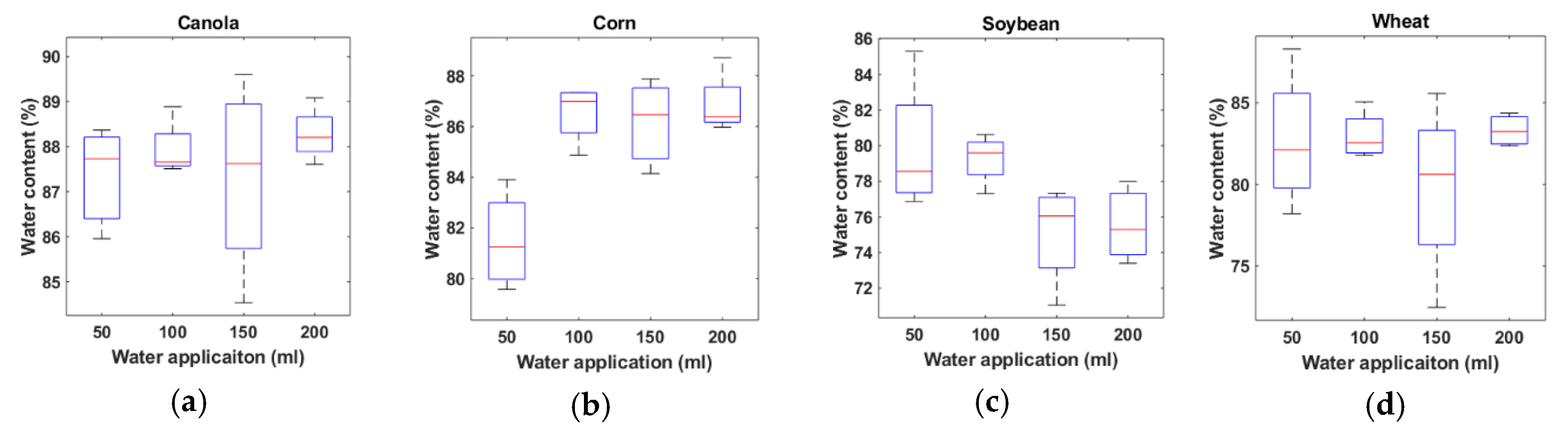
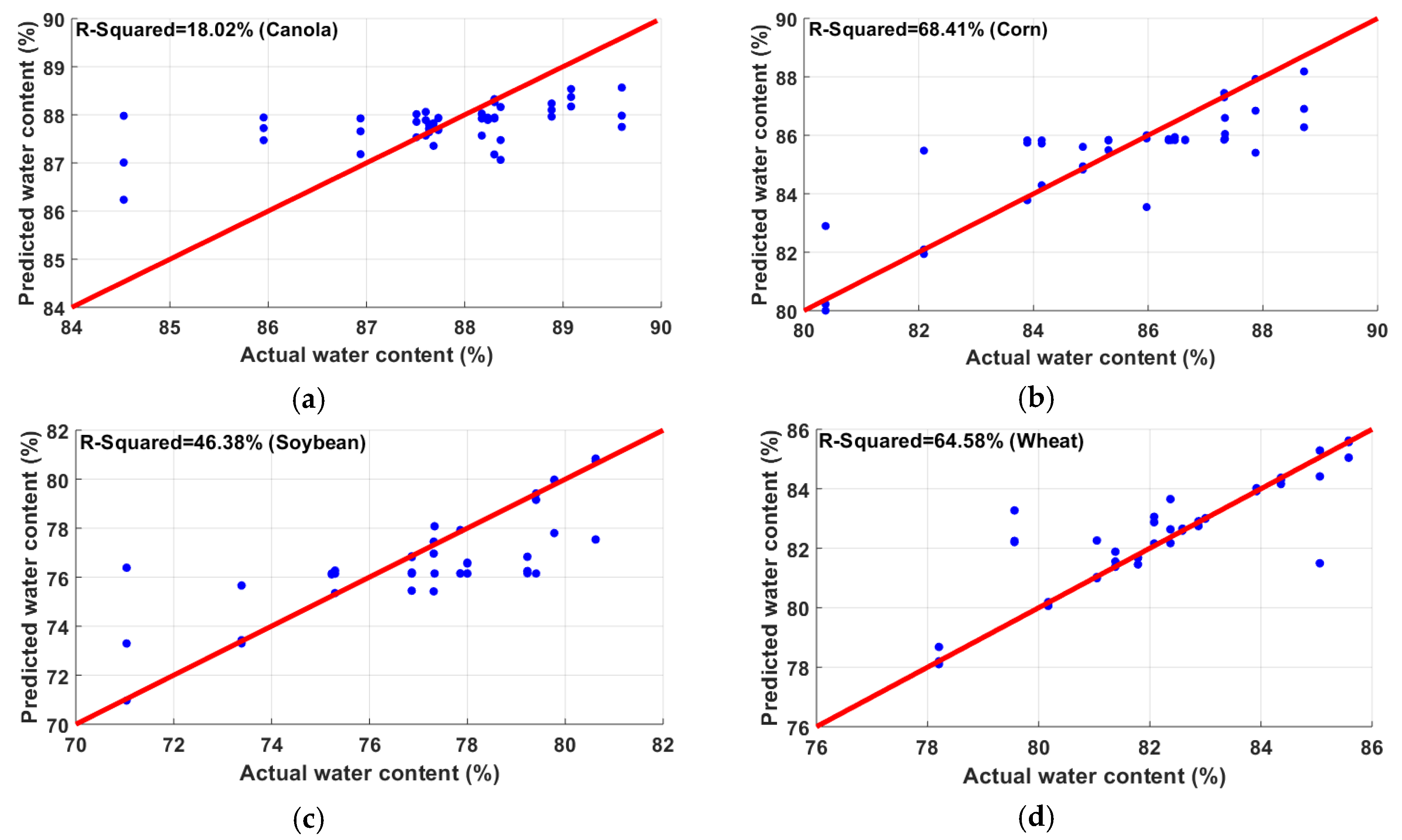
3.2. Water Content Estimation
3.3. Important Wavelengths
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques–A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Sinfield, J.V.; Fagerman, D.; Colic, O. Evaluation of sensing technologies for on-the-go detection of macro-nutrients in cultivated soils. Comput. Electron. Agric. 2010, 70, 1–18. [Google Scholar] [CrossRef]
- Vigneau, N.; Ecarnot, M.; Rabatel, G.; Roumet, P. Potential of field hyperspectral imaging as a non destructive method to assess leaf nitrogen content in wheat. Field Crop. Res. 2011, 122, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, X.; Zhang, H.; Christie, P.; Li, X.; Horlacher, D.; Liebig, H.-P. Evaluation of current fertilizer practice and soil fertility in vegetable production in the Beijing region. Nutr. Cycl. Agroecosyst. 2004, 69, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Liu, Y.; Wu, H.; Zhang, P.; Yu, N.; Zhang, Y.; Zou, H.; Fan, Q.; Zhang, Y. Interactive effects of irrigation and nitrogen fertilizer on yield, nitrogen uptake, and recovery of two successive Chinese cabbage crops as assessed using 15N isotope. Sci. Hortic. 2017, 215, 117–125. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Detection of phosphorus and nitrogen deficiencies in corn using spectral radiance measurements. Agron. J. 2002, 94, 1215. [Google Scholar] [CrossRef] [Green Version]
- Wahono, W.A.; Indradewa, D.; Sunarminto, B.H.; Haryono, E.; Prajitno, D. Evaluation of the use of SPAD-502 chlorophyll meter for non-destructive estimation of nitrogen status of tea leaf (Camellia sinensis L. Kuntze). Indian J. Agric. Res. 2019, 53, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Tafolla, R.O.; Juarez-Lopez, P.; Ontiveros-Capurata, R.E.; Sandoval-Villa, M.; Alia-Tejacal, I.; Alejo-Santiago, G. Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and atLEAF readings. Not. Bot. Horti Agrobot. 2019, 47, 751–756. [Google Scholar]
- Wang, J.; Shen, C.; Liu, N.; Jin, X.; Fan, X.; Dong, C.; Xu, Y. Non-destructive evaluation of the leaf nitrogen concentration by in-field visible/near-infrared spectroscopy in Pear Orchards. Sensors 2017, 17, 538. [Google Scholar] [CrossRef] [Green Version]
- Basyouni, R.; Dunn, B.; Goad, C. Use of nondestructive sensors to assess nitrogen status in potted Dianthus (Dianthus chinensis L.) production. Can. J. Plant Sci. 2016, 97, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crop. Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhu, H.; Li, Z.; Yang, G. Quantitative analysis and hyperspectral remote sensing of the nitrogen nutrition index in winter wheat. Int. J. Remote Sens. 2019, 41, 858–881. [Google Scholar] [CrossRef]
- Yu, K.Q.; Zhao, Y.R.; Li, X.L.; Shao, Y.N.; Liu, F.; He, Y. Hyperspectral imaging for mapping of total nitrogen spatial distribution in pepper plant. PLoS ONE 2014, 9, e116205. [Google Scholar] [CrossRef]
- Ni, C.; Wang, D.; Tao, Y. Variable weighted convolutional neural network for the nitrogen content quantization of Masson pine seedling leaves with near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 32–39. [Google Scholar] [CrossRef]
- Blackmer, T.; Schepers, J.S. Techniques for monitoring crop nitrogen status in corn. Commun. Soil Sci. Plant Anal. 1994, 25, 1791–1800. [Google Scholar] [CrossRef]
- Kusnierek, K.; Korsaeth, A. Simultaneous identification of spring wheat nitrogen and water status using visible and near infrared spectra and powered partial least squares regression. Comput. Electron. Agric. 2015, 117, 200–213. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crop. Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Wang, S.; Ma, Y.; Wei, Q.; Li, X.; Cong, R.; Ren, T. Methods for estimating leaf nitrogen concentration of winter oilseed rape (Brassica napus L.) using in situ leaf spectroscopy. Ind. Crops Prod. 2016, 91, 194–204. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; He, Y.; Gong, X. Detecting macronutrients content and distribution in oilseed rape leaves based on hyperspectral imaging. Biosyst. Eng. 2013, 115, 56–65. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using near- and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Jin, X.; Shi, C.; Yu, C.Y.; Yamada, T.; Sacks, E.J. Determination of leaf water content by visible and near-infrared spectrometry and multivariate calibration in Miscanthus. Front. Plant Sci. 2017, 8, 721. [Google Scholar] [CrossRef] [Green Version]
- Gente, R.; Born, N.; Voß, N.; Sannemann, W.; Léon, J.; Koch, M.; Castro-Camus, E. Determination of leaf water content from terahertz time-domain spectroscopic data. J. Infrared Millim. Terahertz Waves 2013, 34, 316–323. [Google Scholar] [CrossRef]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High throughput in vivo analysis of plant leaf chemical properties using hyperspectral imaging. Front. Plant Sci. 2017, 8, 1348. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Q.; Zhang, G. Rapid determination of leaf water content using VIS/NIR spectroscopy analysis with wavelength selection. Spectrosc. An Int. J. 2012, 27, 93–105. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Gopalakrishnan, K.; Satish, K. Embedded image capturing system using raspberry pi system. Int. J. Emerg. Trends Technol. Comput. Sci. 2014, 3, 213–215. [Google Scholar]
- Imteaj, A.; Rahman, T.; Hossain, M.K.; Alam, M.S.; Rahat, S.A. An IoT based fire alarming and authentication system for workhouse using Raspberry Pi 3. In Proceedings of the 2017 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’s Bazar, Bangladesh, 16–18 February 2017; pp. 899–904. [Google Scholar]
- Paper Mirror. Available online: https://www.amazon.ca/Cloakroom-Decorative-Background-Removable-Self-Adhesive/dp/B07W312F98/ref=asc_df_B07W312F98/?tag=googleshopc0c-20&linkCode=df0&hvadid=335179893508&hvpos=1o2&hvnetw=g&hvrand=6497894200531816537&hvpone=&hvptwo=&hvqmt=&hvdev=c&hvdvcmdl= (accessed on 4 February 2020).
- Mohebian, M.R.; Marateb, H.R.; Mansourian, M.; Mañanas, M.A.; Mokarian, F. A hybrid computer-aided-diagnosis system for prediction of breast cancer recurrence (HPBCR) using optimized ensemble learning. Comput. Struct. Biotechnol. J. 2017, 15, 75–85. [Google Scholar] [CrossRef]
- Boneau, C.A. The effects of violations of assumptions underlying the t test. Psychol. Bull. 1960, 57, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Firouzabadi, F.S.; Vard, A.; Sehhati, M.; Mohebian, M. An optimized framework for cancer prediction using immunosignature. J. Med. Signals Sens. 2018, 8, 161–169. [Google Scholar]
- Analytical Methods Committee. Robust statistics, how not to reject outliers. Analyst 1989, 114, 1693–1697. [Google Scholar] [CrossRef]
- Seeger, M. Gaussian processes for machine learning. Int. J. Neural Syst. 2004, 14, 69–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wang, B. Bayesian variable selection for Gaussian process regression: Application to chemometric calibration of spectrometers. Neurocomputing 2010, 73, 2718–2726. [Google Scholar] [CrossRef] [Green Version]
- Lunderman, S.; Fioroni, G.M.; McCormick, R.L.; Nimlos, M.R.; Rahimi, M.J.; Grout, R.W. Screening fuels for autoignition with small-volume experiments and gaussian process classification. Energy Fuels 2018, 32, 9581–9591. [Google Scholar] [CrossRef]
- Lin, M.; Song, X.; Qian, Q.; Li, H.; Sun, L.; Zhu, S.; Jin, R. Robust gaussian process regression for real-time high precision GPS signal enhancement. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining KDD’19, Anchorage, AK, USA, 25 July 2019; pp. 2838–2847. [Google Scholar]
- Ross, K.A.; Jensen, C.S.; Snodgrass, R.; Dyreson, C.E.; Jensen, C.S.; Snodgrass, R.; Skiadopoulos, S.; Sirangelo, C.; Larsgaard, M.L.; Grahne, G. Cross-Validation. In Encyclopedia of Database Systems; Springer: Boston, MA, USA, 2009; pp. 532–538. [Google Scholar]
- Botchkarev, A. Performance metrics (error measures) in machine learning regression, forecasting and prognostics: Properties and typology. Interdiscip. J. Inf. Knowl. Manag. 2018, 14, 045–076. [Google Scholar]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in Sweet Pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [Green Version]
- Li, J.W.; Zhang, J.X.; Zhao, Z.; Lei, X.D.; Xu, X.L.; Lu, X.X.; Weng, D.L.; Gao, Y.; Cao, L.K. Use of fluorescence-based sensors to determine the nitrogen status of paddy rice. J. Agric. Sci. 2013, 151, 862–871. [Google Scholar] [CrossRef]










| Validation Parameter | Definition |
|---|---|
| Root-mean-square error (RMSE) | |
| Mean squared error (MSE) | |
| Mean absolute error (MAE) | |
| Co-efficient of determination () |
| Plant Species | RMSE | MAE | |
|---|---|---|---|
| Canola | 63.91 | 1.28 | 0.87 |
| Corn | 80.05 | 0.50 | 0.31 |
| Soybean | 82.29 | 0.21 | 0.12 |
| Wheat | 63.21 | 0.57 | 0.37 |
| All crops combined | 73.96 | 1.13 | 0.72 |
| Plant Species | RMSE | MAE | |
|---|---|---|---|
| Canola | 18.02 | 1.06 | 0.76 |
| Corn | 68.41 | 1.17 | 0.75 |
| Soybean | 46.38 | 3.50 | 2.11 |
| Wheat | 64.58 | 1.16 | 0.85 |
| All crops combined | 46.08 | 3.97 | 2.75 |
| Device Components | Approximate Cost (USD) |
|---|---|
| Sensor1 | $25 |
| Sensor2 | $25 |
| MUX | $20 |
| Control circuit (RP3) | $50 |
| Power bank | $10 |
| Display | $5 |
| Manufacturing | $50 |
| Others | $15 |
| Total | $200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibullah, M.; Mohebian, M.R.; Soolanayakanahally, R.; Wahid, K.A.; Dinh, A. A Cost-Effective and Portable Optical Sensor System to Estimate Leaf Nitrogen and Water Contents in Crops. Sensors 2020, 20, 1449. https://doi.org/10.3390/s20051449
Habibullah M, Mohebian MR, Soolanayakanahally R, Wahid KA, Dinh A. A Cost-Effective and Portable Optical Sensor System to Estimate Leaf Nitrogen and Water Contents in Crops. Sensors. 2020; 20(5):1449. https://doi.org/10.3390/s20051449
Chicago/Turabian StyleHabibullah, Mohammad, Mohammad Reza Mohebian, Raju Soolanayakanahally, Khan A. Wahid, and Anh Dinh. 2020. "A Cost-Effective and Portable Optical Sensor System to Estimate Leaf Nitrogen and Water Contents in Crops" Sensors 20, no. 5: 1449. https://doi.org/10.3390/s20051449






