Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose
Abstract
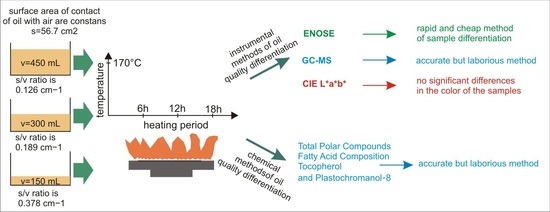
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heating Process
2.3. Total Polar Compound (TPC) Analysis
2.4. Iodine Value Calculation (CIV)
2.5. Fatty Acid Composition Analysis
2.6. Tocopherol and Plastochromanol-8 Analysis
2.7. Imaging Colorimeter
2.8. Electronic Nose
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.10. Principal Component Analysis (PCA)
3. Results and Discussion
3.1. Total Polar Compound Content (TPC) and Fatty Acid Composition
3.2. Imaging Colorimeter
3.3. Identification of Major Volatile Compounds—GC-MS Analysis
3.4. Electronic Nose Responses
3.5. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kus-Yamashita, M.; Filho, J.; Mcdonald, B.; Ravacci, G.; Rogero, M.; Santos, R.; Waitzberg, D.; Reyes, M.; Yehuda, S.; Gierke, J.; et al. Polyunsaturated fatty acids: Health impacts. Eur. J. Nutr. Food Saf. 2016, 6, 111–131. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of dietary fats on brain functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- Zaunschirm, M.; Pignitter, M.; Kienesberger, J.; Hernler, N.; Riegger, C.; Eggersdorfer, M.; Somoza, V. Contribution of the ratio of tocopherol homologs to the oxidative stability of commercial vegetable oils. Molecules 2018, 23, 206. [Google Scholar] [CrossRef] [Green Version]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Raczyk, M.; Kmiecik, D.; Przybylski, R.; Rudzińska, M. Effect of fatty acid unsaturation on phytosteryl ester degradation. J. Am. Oil Chem. Soc. 2017, 94, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kulczyński, B. Degradation of tocopherol molecules and its impact on the polymerization of triacylglycerols during heat treatment of oil. Molecules 2019, 24, 4555. [Google Scholar] [CrossRef] [Green Version]
- Gertz, C. Fundamentals of the frying process. Eur. J. Lipid Sci. Technol. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Ziaiifar, A.M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 2008, 43, 1410–1423. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Urgeghe, P.; Ferro, M.; Del Caro, A.; Taras, A.; Garroni, S.; Rourke, J.P.; Cabizza, R.; Petretto, G.L. Variation of the chemical composition of waste cooking oils upon bentonite filtration. Resources 2019, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Alvis, A.; Vélez, C.; Rada-Mendoza, M.; Villamiel, M.; Villada, H.S. Heat transfer coefficient during deep-fat frying. Food Control 2009, 20, 321–325. [Google Scholar] [CrossRef]
- Falade, A.O.; Oboh, G.; Okoh, A.I. Potential health implications of the consumption of thermally-oxidized cooking oils–a review. Polish J. Food Nutr. Sci. 2017, 67, 95–105. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, M.; Hassanein, M.M.M.; Abdel-Razek, A.G.; Kmiecik, D.; Siger, A.; Ratusz, K. Influence of composition on degradation during repeated deep-fat frying of binary and ternary blends of palm, sunflower and soybean oils with health-optimised saturated-to-unsaturated fatty acid ratios. Int. J. Food Sci. Technol. 2018, 53, 1021–1029. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Siger, A.; Rusinek, R. Degradation of tocopherols during rapeseed storage in simulated conditions of industrial silos. Int. Agrophysics 2016, 30. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Technol. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Xu, M.L.; Zhu, S.M.; Yu, Y. Quality assessment of Chinese liquor with different ages and prediction analysis based on gas chromatography and electronic nose. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Multari, S.; Marsol-vall, A.; Heponiemi, P.; Suomela, J.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Li, A.; Duan, S.; Dang, Y.; Zhang, X.; Xia, K.; Liu, S.; Han, X.; Wen, J.; Li, Z.; Wang, X.; et al. Origin identification of Chinese Maca using electronic nose coupled with GC-MS. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, M.; Nawrocka, A.; Rusinek, R. Identification of Volatile Organic Compounds and Their Concentrations Using a Novel Method Analysis of MOS Sensors Signal. J. Food Sci. 2019, 84. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, R.; Jelen, H.; Malaga-Toboła, U.; Molenda, M.; Gancarz, M. Influence of changes in the level of volatile compounds emitted during rapeseed quality degradation on the reaction of MOS type sensor-array. Sensors 2020, 20, 3135. [Google Scholar] [CrossRef] [PubMed]
- AOCS. AOCS Official Method 982.27. Polar Components in Frying Fats, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Siger, A.; Michalak, M.; Rudzińska, M. Canolol, tocopherols, plastochromanol-8, and phytosterols content in residual oil extracted from rapeseed expeller cake obtained from roasted seed. Eur. J. Lipid Sci. Technol. 2016, 118, 1358–1367. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and differentiation of volatile compound profiles in roasted coffee arabica beans from different countries using an electronic nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Dobarganes, M.C.; Márquez-Ruiz, G. Regulation of used frying fats and validity of quick tests for discarding the fats. Grasas y Aceites 1998, 49, 331–335. [Google Scholar] [CrossRef]
- Alzaa, D.F.; Guillaume, C.; Ravetti, L. Evaluation of Chemical and Physical Changes in Different Commercial Oils during Heating. Acta Sci. Nurtitional Heal 2018, 2, 2–11. [Google Scholar]
- Aladedunye, F.; Przybylski, R. Frying stability of high oleic sunflower oils as affected by composition of tocopherol isomers and linoleic acid content. Food Chem. 2013, 141, 2373–2378. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Adv. Colloid Interface Sci. 2006, 128, 267–272. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Litwinienko, G. Autooxidation of unsaturated fatty acids and their esters. J. Therm. Anal. Calorim. 2001, 65, 639–646. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Ravi, R.; Prakash, M.; Bhat, K.K. Sensory odour profiling and physical characteristics of edible oil blends during frying. Food Res. Int. 2005, 38, 59–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Chang, C.; Lv, Y.; Lai, W.; Zhang, H.; Wang, X.; Jin, Q. Determination of Origin of Commercial Flavored Rapeseed Oil by the Pattern of Volatile Compounds Obtained via GC–MS and Flash GC Electronic Nose. Eur. J. Lipid Sci. Technol. 2020, 122, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Chen, J.; Jing, B.; Zhang, L.; Yu, X. Characterization of Differences in Flavor in Virgin Rapeseed Oils by Using Gas Chromatography–Mass Spectrometry, Electronic Nose, and Sensory Analysis. Eur. J. Lipid Sci. Technol. 2020, 122, 1–10. [Google Scholar] [CrossRef]
- Torri, L.; Bondioli, P.; Folegatti, L.; Rovellini, P.; Piochi, M.; Morini, G. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem. 2019, 286, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.D.; Moser, H.A.; List, G.R. Odor and flavor responses to additives in edible oils. J. Am. Oil Chem. Soc. 1971, 48, 495–498. [Google Scholar] [CrossRef]
- Asikin, Y.; Maeda, G.; Tamaki, H.; Mizu, M.; Oku, H.; Wada, K. Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. FRIN 2015, 67, 102–110. [Google Scholar] [CrossRef]
- Garcia, R.; Martins, N.; Cabrita, M.J. Putative markers of adulteration of extra virgin olive oil with re fi ned olive oil: Prospects and limitations. FRIN 2013, 6–11. [Google Scholar] [CrossRef]






| Not Heated | 0.378 | 0.189 | 0.126 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 18 h | 6 h | 12 h | 18 h | 6 h | 12 h | 18 h | ||
| Fatty Acid Composition | ||||||||||
| C 16:0 | 4.76 ± 0.00 | 5.24 ± 0.07 | 5.34 ± 0.14 | 5.47 ± 0.02 | 4.89 ± 0.03 | 5.04 ± 0.07 | 5.15 ± 0.10 | 4.71 ± 0.06 | 4.77 ± 0.10 | 4.86 ± 0.17 |
| C 18:0 | 1.56 ± 0.01 | 1.53 ± 0.30 | 1.76 ± 0.00 | 1.86 ± 0.01 | 1.57 ± 0.06 | 1.66 ± 0.02 | 1.75 ± 0.00 | 1.61 ± 0.01 | 1.65 ± 0.00 | 1.64 ± 0.05 |
| C 18:1 | 63.83 ± 0.02 | 67.17 ± 0.30 | 67.84 ± 0.27 | 70.13 ± 0.34 | 65.52 ± 0.28 | 66.88 ± 0.01 | 67.82 ± 0.18 | 65.16 ± 0.08 | 66.08 ±0.00 | 66.75 ± 0.08 |
| C 18:2 | 19.07 ± 0.03 | 17.22 ± 0.05 | 16.73 ± 0.58 | 15.13 ± 0.20 | 18.21 ± 0.07 | 17.46 ± 0.04 | 16.78 ± 0.06 | 18.42 ± 0.04 | 17.97 ± 0.07 | 17.58 ± 0.06 |
| C 18:3 | 8.29 ± 0.06 | 6.22 ± 0.15 | 5.66 ± 0.19 | 4.65 ± 0.19 | 7.40 ± 0.21 | 6.48 ± 0.07 | 5.92 ± 0.06 | 7.63 ± 0.03 | 7.05 ± 0.02 | 6.63 ± 0.18 |
| C 20:0 | 0.47 ± 0.01 | 0.54 ± 0.02 | 0.56 ± 0.02 | 0.62 ± 0.01 | 0.51 ± 0.02 | 0.53 ± 0.01 | 0.56 ± 0.02 | 0.50 ± 0.01 | 0.51 ± 0.01 | 0.54 ± 0.01 |
| C 20:1 | 1.66 ± 0.06 | 1.69 ± 0.03 | 1.66 ± 0.05 | 1.75 ± 0.04 | 1.53 ± 0.06 | 1.59 ± 0.06 | 1.65 ± 0.03 | 1.56 ± 0.06 | 1.65 ± 0.03 | 1.62 ± 0.08 |
| C 22:1 | 0.26 ± 0.00 | 0.28 ± 0.02 | 0.30 ± 0.04 | 0.29 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.03 | 0.29 ± 0.03 | 0.27 ± 0.03 | 0.22 ± 0.09 | 0.29 ± 0.02 |
| C 24:0 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.11 ± 0.05 | 0.11 ± 0.03 | 0.09 ± 0.01 | 0.09 ± 0.04 | 0.14 ± 0.04 | 0.09 ± 0.07 | 0.10 ± 0.01 |
| SFA | 6.90± 0.04 | 7.43 ± 0.26 | 7.81 ± 0.15 | 8.05 ± 0.04 | 7.09 ± 0.04 | 7.32 ± 0.06 | 7.54 ± 0.13 | 6.96 ± 0.06 | 7.02 ± 0.17 | 7.13 ± 0.21 |
| MUFA | 65.74± 0.04 | 69.14 ± 0.27 | 69.80 ± 0.34 | 72.17 ± 0.35 | 67.30 ± 0.29 | 68.74 ± 0.08 | 69.76 ± 0.20 | 66.99 ± 0.02 | 67.96 ± 0.12 | 68.66 ± 0.01 |
| PUFA | 27.36 ± 0.08 | 23.43 ± 0.15 | 22.39± 0.48 | 19.77 ± 0.37 | 25.61 ± 0.27 | 23.94 ± 0.07 | 22.70 ± 0.11 | 26.04 ± 0.07 | 25.03 ± 0.05 | 24.21 ± 0.22 |
| CIV | 111.09 ± 0.16 | 105.37 ± 0.40 | 103.65 ± 0.50 | 100.25 ± 0.51 | 108.63 ± 0.40 | 106.15 ± 0.14 | 104.37 ± 0.13 | 109.31 ± 0.14 | 107.87 ± 0.15 | 106.68 ± 0.53 |
| Total chromanols * | 82.14 ± 0.56 | 4.10 ± 0.35 | 0.33 ± 0.04 | 0.03 ± 0.03 | 30.60 ± 1.78 | 5.53 ± 0.55 | 1.42 ± 0.26 | 49.35 ± 1.21 | 24.36 ± 2.79 | 2.80 ± 0.47 |
| Volatile Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Not Heated (1) | 6 h 0.378 s/v (2) | 12 h 0.378 s/v (3) | 18 h 0.378 s/v (4) | 6 h 0.189 s/v (5) | 12 h 0.189 s/v (6) | 18 h 0.189 s/v (7) | 6 h 0.126 s/v (8) | 12 h 0.126 s/v (9) | 18 h 0.126 s/v (10) |
| 1.2 * 4(3H)-quinazolinone, 2-methyl-3-(2-methylphenyl) | 1.2 * acetic acid, trichloro | 1.2 *N,N-dimethyl-2H-pyran-2-iminium chloride | 1.2 *N,N-dimethyl-2H-pyran-2-iminium chloride | ||||||
| 1.8 * phenol,3-methyl-4-nitro-, benzenesulfonate (ester) | 1.8 * 2-octadec-1-enyloxy-1,1,2,2-tetradeutero ethanol | 1.8 * 2-octadec-1-enyloxy-1,1,2,2-tetradeutero ethanol | 1.8 * 2-octadec-1-enyloxy-1,1,2,2-tetradeutero ethanol | 1.8 * 3,4-dihydrothienyl-(3,4,β)-5-carboxythiol | 1.8 * pentasiloxane,1,2,3,3,5,5,7,7,9,9-decamethyl | 1.8 * 2-penten-1-ol | 1.8 * 2h-pyrrole,2,2-dimethyl-3,5-diphenyl,1-oxide | 1.8 * 2-octadec-1-enyloxy-1,1,2,2-tetradeutero ethanol | 1.8 * methyl-d3 1-diderterio-2-propenyl ether |
| 3.0 * 4-methoxy-2-(1-phenyletheny)phenol | 3.0 * 2-[4-(benzyloxy)phenoxy]-n-(2-nitrophenyl)acetamide | 3.0 * 2-[4-(benzyloxy)phenoxy]-n-(2-nitrophenyl)acetamide | 3.0 * 2-thiopheneethanol,5-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)- | 3.0 * 2-thiopheneethanol,5-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)- | |||||
| 4.8 * 1h-indole,5-chloro- | 4.8 * cyano-[5-(2-hydroxy-acetyl)-pyrrolidin-2-ylidene]-acetic acid tert-butyl ester | ||||||||
| 8.1 * benzenamine, n-(10,11-dihydro-5h-dibenso[a,d]cyclohepten-5-ylidene-,n-oxide | 8.1 * 3,4-pyridinedicarboxylic acid,6-(diethylamino)-2-methoxy-,dimethyl ester | 8.1 * 3,4-pyridinedicarboxylic acid,6-(diethylamino)-2-methoxy-,dimethyl ester | |||||||
| 8.4 * 3,4-pyridinedicarboxylic acid,6-(diethylamino)-2-methoxy-,dimethyl ester | 8.4 * 3(5)-(4-chlorophenyl)-4-nitroso-5(3)-phenylaminopyrazole | 8.4 * (1,3-dimethyl-2-methylene-cyclopentyl)-methanol | 8.4 * 1-(2,3-dimethylphenyl)anthracene | 8.4 * 1-(2,3-dimethylphenyl)anthracene | 8.4 * 1-(2,3-dimethylphenyl)anthracene | ||||
| 9.0 * 6,9,12-octadecatrienoic acid, methyl ester | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal, | 9.0 * 2,4-heptadienal | 9.0 * 2,4-heptadienal, |
| 10.8 * cis-9,10-dimethylanthracene-9-10-diol | |||||||||
| 12.0 * 4,5-secocholest-8-en-3-one, 5-ethylidene-14-methyl | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne | 12.0 * 1-decyne |
| 17.6 * 13-heptadecyn-1-ol | |||||||||
| 18.0 * 2-methyl-3-ethoxycarbonyl-4-phenyl-5-oxoindeno [1,2-b]pyridine oxime | 18.4 * cycloheptane-pentanoic acid, 1-nitro-β,2-dioxo,phenylmethyl ester | 18.4 * endo-dicyclopentadiene dioxide | 18.4 * tricyclo[6,3,3,0] tetradec-4-ene-10-dione | 18.4 * 2,4-nonadienal, | 18.4 * 2,4-nonadienal, | 18.4 * 2,4-nonadienal, | 18.4 * 2,4-nonadienal, | 18.4 * 2,4-nonadienal, | 18.4 * 2,4-nonadienal |
| 26.0 * 2,3-diethyl-1,5,7-trimethoxyindenone | 26.0 * 1,2-benzenediccarboxylic acid, diethyl ester | ||||||||
| 31.5 * stearic acid, 3-(octadecyloxy)propyl ester | 31.5 * 1,2-benzenediccarboxylic acid, butyl octyl ester | 31.5 * 2,7-diphenyl-1,6-dioxopyridazino[4,5-2,3]pyrrolo[4,5-δ]pyridazine | 31.5 * 4h-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxy | ||||||
| TGS2602 | AS-MLV-P2 | TGS2603 | TGS2612 | TGS2610 | TGS2611 | TGS2620 | TGS2600 | |
|---|---|---|---|---|---|---|---|---|
| ΔR/Rmax | ||||||||
| Not heated (1) | 0.17 ± 0.01 | 0.025 ± 0.0 | 0.08 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.00 |
| 6 h 0.378 s/v (2) | 0.5 ± 0.01 | 0.22 ± 0.01 | 0.3 ± 0.01 | 0.01 ± 0.00 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 |
| 12 h 0.378 s/v (3) | 0.8 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.1 ± 0.00 | 0.2 ± 0.01 | 0.19 ± 0.01 | 0.32 ± 0.01 | 0.37 ± 0.01 |
| 18 h 0.378 s/v (4) | 1.11 ± 0.03 | 0.57 ± 0.02 | 0.5 ± 0.02 | 0.03 ± 0.00 | 0.4 ± 0.01 | 0.37 ± 0.02 | 0.82 ± 0.03 | 0.87 ± 0.02 |
| 6 h 0.189 s/v (5) | 0.52 ± 0.02 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.02 ± 0.00 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.22 ± 0.01 |
| 12 h 0.189 s/v (6) | 0.65 ± 0.01 | 0.34 ± 0.01 | 0.3 ± 0.01 | 0.02 ± 0.00 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.39 ± 0.01 | 0.43 ± 0.01 |
| 18 h 0.189 s/v (7) | 0.77 ± 0.01 | 0.36 ± 0.01 | 0.33 ± 0.01 | 0.02 ± 0.00 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.44 ± 0.02 | 0.5 ± 0.02 |
| 6 h 0.126 s/v (8) | 0.6 ± 0.01 | 0.34 ± 0.01 | 0.25 ± 0.01 | 0.015 ± 0.00 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.42 ± 0.02 | 0.45 ± 0.02 |
| 12 h 0.126 s/v (9) | 0.92 ± 0.02 | 0.41 ± 0.01 | 0.61 ± 0.02 | 0.015 ± 0.00 | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.55 ± 0.02 | 0.6 ± 0.02 |
| 18 h 0.126 s/v (10) | 0.94 ± 0.02 | 0.45 ± 0.02 | 0.4 ± 0.02 | 0.015 ± 0.00 | 0.3 ± 0.01 | 0.28 ± 0.01 | 0.8 ± 0.02 | 0.86 ± 0.02 |
| tRatio | ||||||||
| Not heated (1) | 0.24 ± 0.01 | 0.24 ± 0.02 | 0.24 ± 0.01 | 0.24 ± 0.02 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.24 ± 0.02 | 0.24 ± 0.02 |
| 6 h 0.378 s/v (2) | 0.2 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 |
| 12 h 0.378 s/v (3) | 0.2 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| 18 h 0.378 s/v (4) | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.00 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| 6 h 0.189 s/v (5) | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.11 ± 0.01 | 0.11 ± 0.00 |
| 12 h 0.189 s/v (6) | 0.2 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| 18 h 0.189 s/v (7) | 0.2 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.00 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| 6 h 0.126 s/v (8) | 0.16 ± 0.01 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.04 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.11 ± 0.01 | 0.11 ± 0.01 |
| 12 h 0.126 s/v (9) | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| 18 h 0.126s/v (10) | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.00 | 0.17 ± 0.01 | 0.16 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinek, R.; Kmiecik, D.; Gawrysiak-Witulska, M.; Malaga-Toboła, U.; Tabor, S.; Findura, P.; Siger, A.; Gancarz, M. Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose. Sensors 2021, 21, 303. https://doi.org/10.3390/s21010303
Rusinek R, Kmiecik D, Gawrysiak-Witulska M, Malaga-Toboła U, Tabor S, Findura P, Siger A, Gancarz M. Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose. Sensors. 2021; 21(1):303. https://doi.org/10.3390/s21010303
Chicago/Turabian StyleRusinek, Robert, Dominik Kmiecik, Marzena Gawrysiak-Witulska, Urszula Malaga-Toboła, Sylwester Tabor, Pavol Findura, Aleksander Siger, and Marek Gancarz. 2021. "Identification of the Olfactory Profile of Rapeseed Oil as a Function of Heating Time and Ratio of Volume and Surface Area of Contact with Oxygen Using an Electronic Nose" Sensors 21, no. 1: 303. https://doi.org/10.3390/s21010303