A Preliminary Study on Hepatoprotective, Hypolipidemic and Aortic Morphometric Effects of Omega-3-Rich Fish Oil in Hypercholesterolemic Mice
Abstract
:1. Introduction
2. Results
2.1. Weight Gain and Food Intake of the Treatment Groups
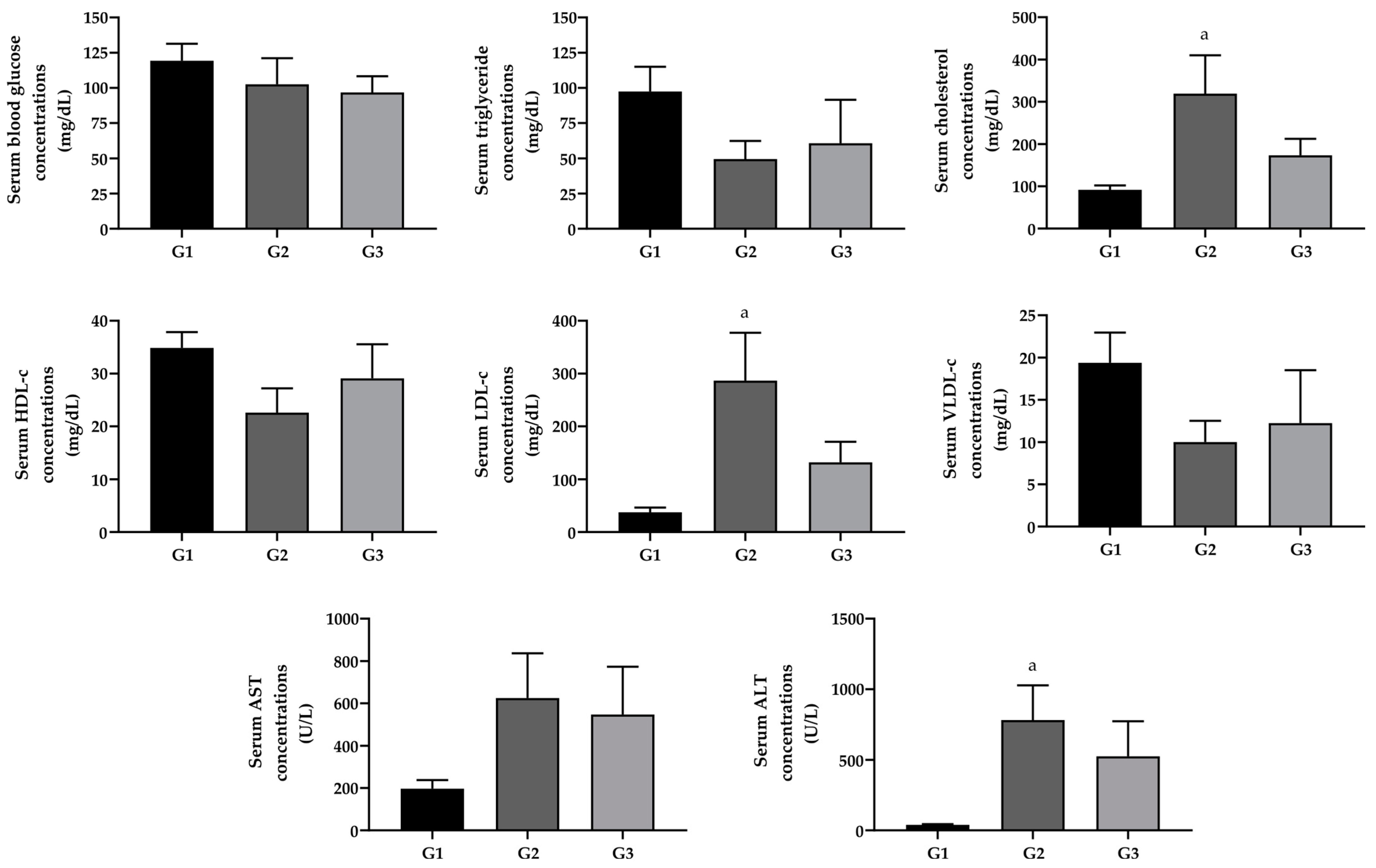
2.2. Effect of Omega-3 Fatty Acid Supplementation on Blood Glucose, Lipid Profile, and Liver Cytolytic Enzymes
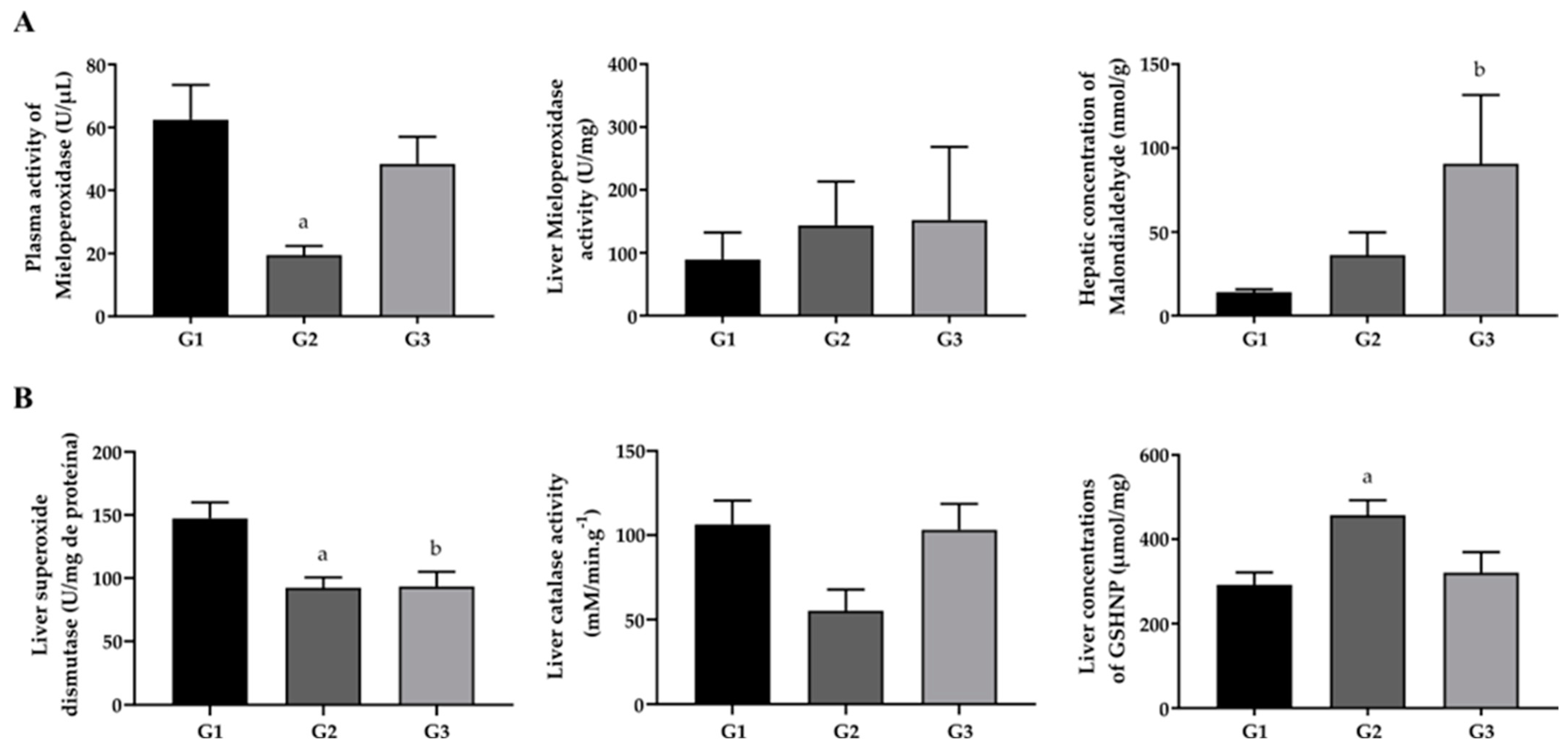
2.3. Effect of Omega-3 Fatty Acid Supplementation on Oxidative Stress Biomarkers
2.4. Effect of Omega-3 Fatty Acid Supplementation on the Lipid Content of Liver Tissue
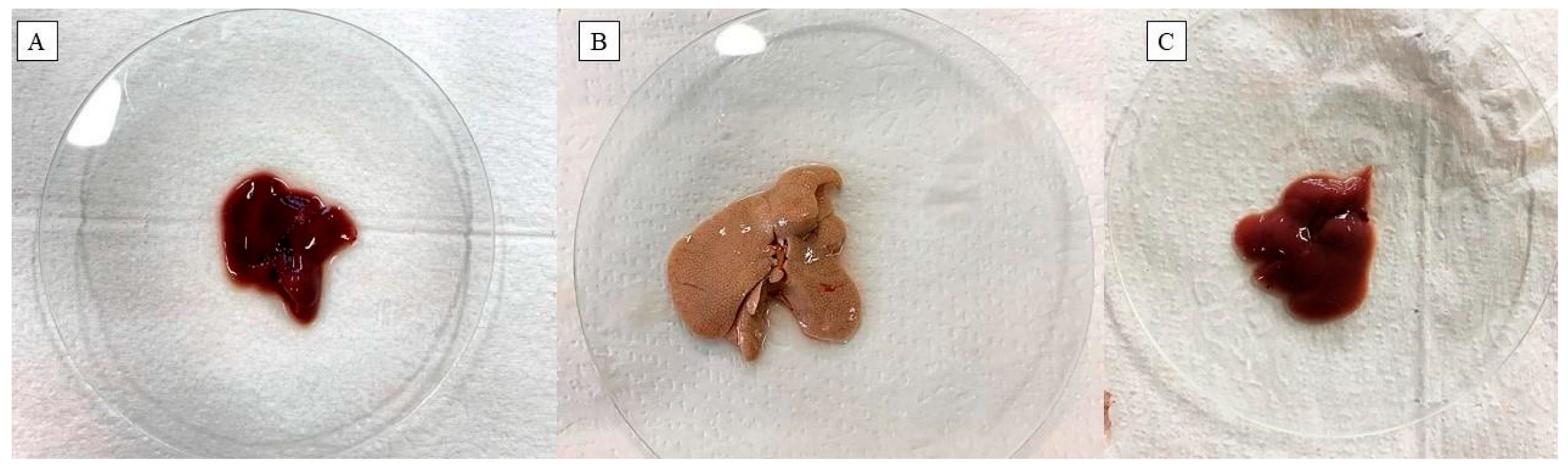
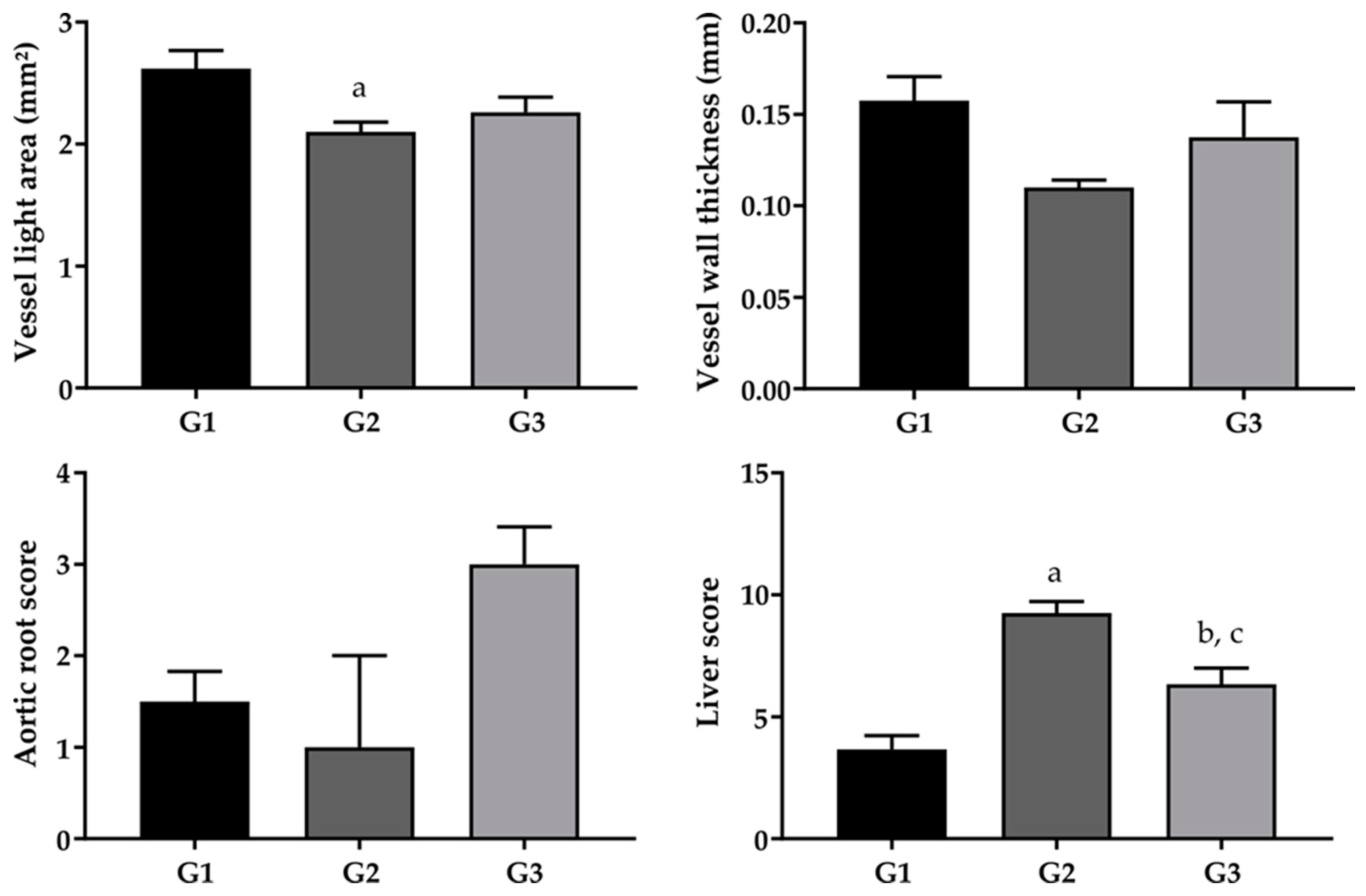
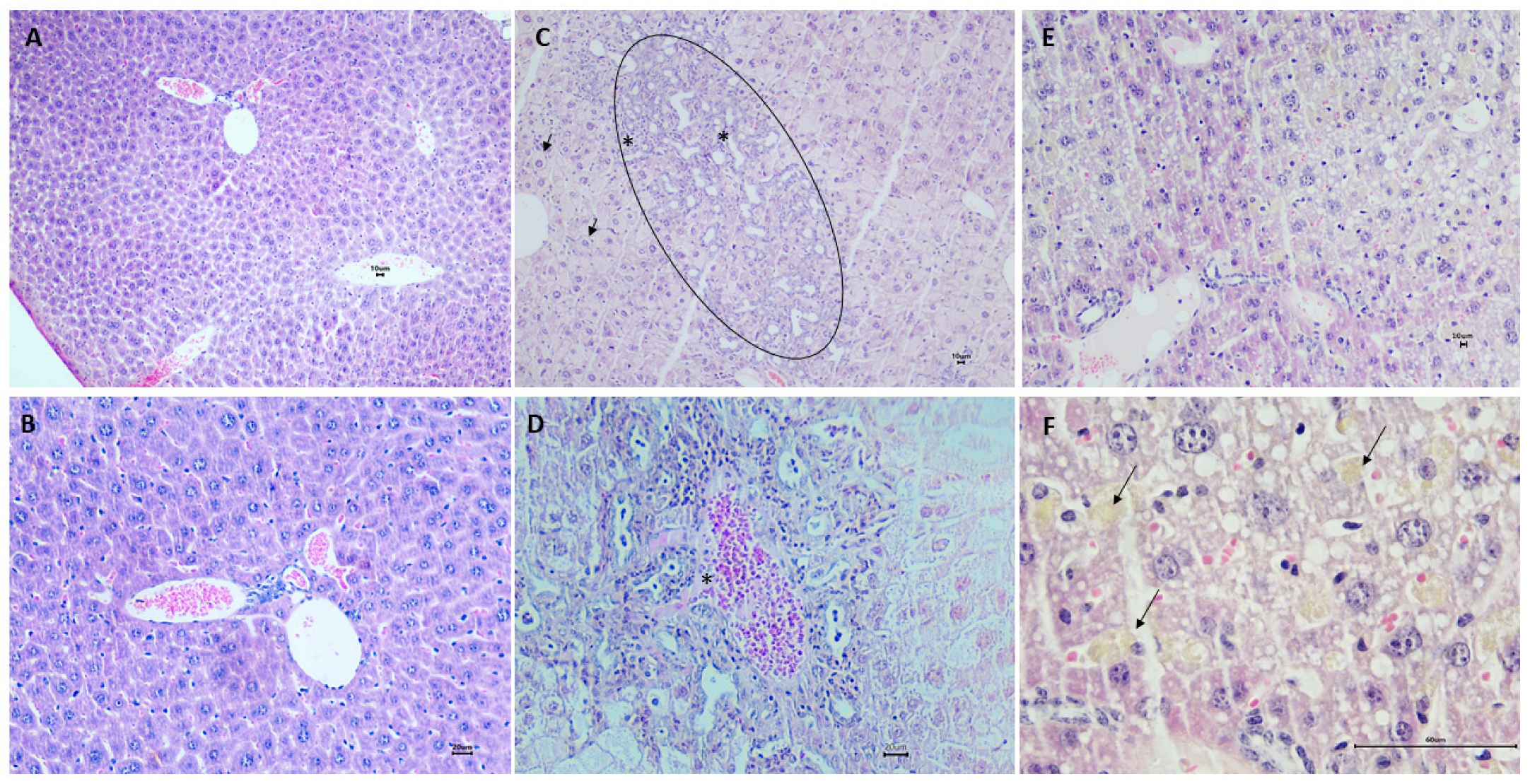
2.5. Effect of Fish Oil Supplementation on the Morphological, Morphometric and Histological Aspects of the Liver
3. Discussion
4. Materials and Methods
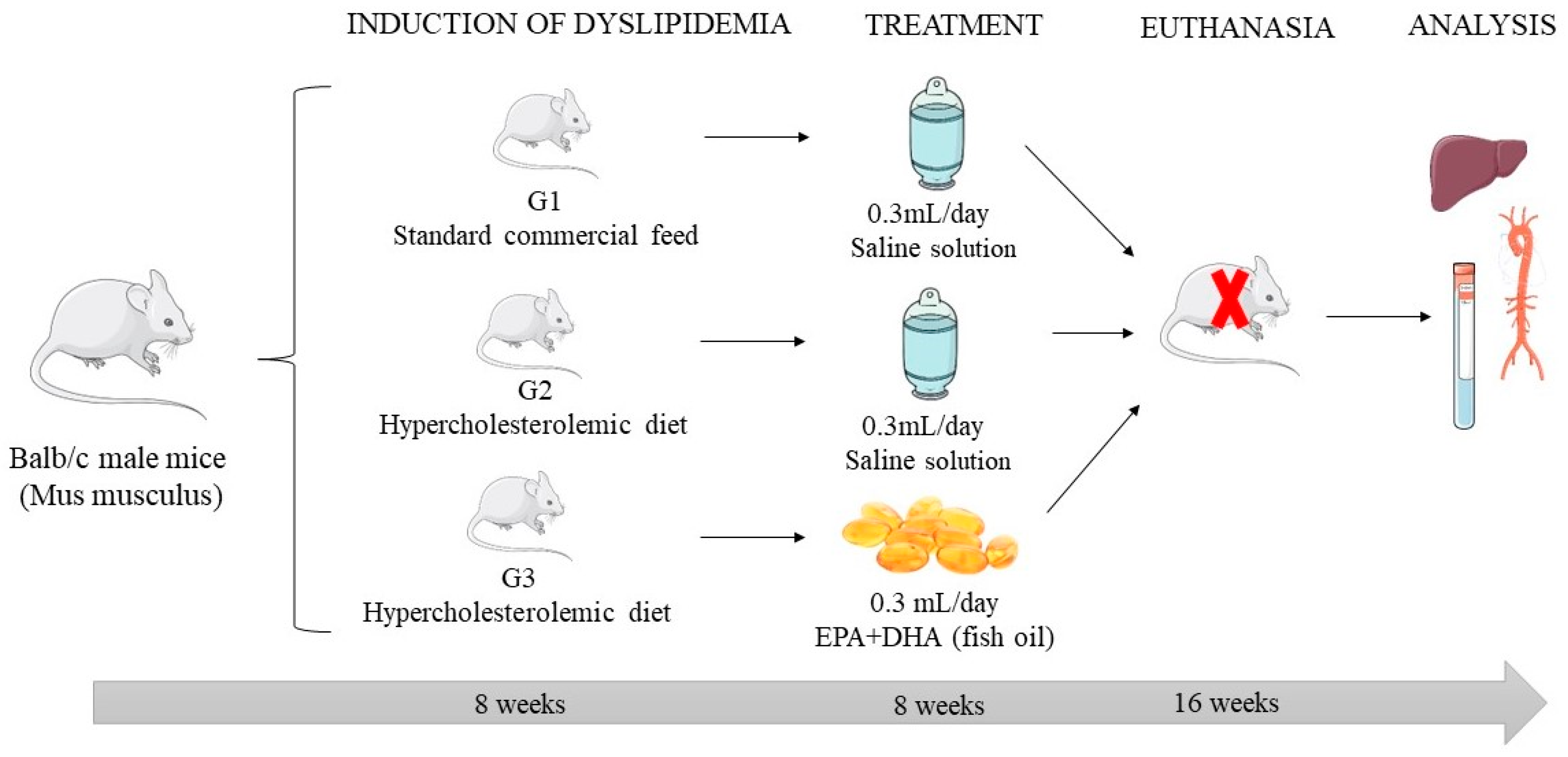
4.1. Experimental Model of Dyslipidemia
4.2. Determination of the Chemical Composition of the Diets
4.3. Assessment of Food Intake and Body Weight
4.4. Blood and Tissue Collection
4.5. Determination of Biochemical Parameters
4.6. Determination of Oxidative Stress Biomarkers
4.6.1. Determination of Myeloperoxidase (MPO) Activity
4.6.2. Determination of the Malondialdehyde (MDA) Concentration
4.7. Determination of the Antioxidant Activity in the Liver
4.7.1. Superoxide Dismutase (SOD) Activity
4.7.2. Catalase Activity (CAT)
4.7.3. Determination of Non-Protein Sulfhydryl Group (GSHNP) Concentrations
4.8. Extraction of Hepatic Lipids and Determination of Lipid Content
4.9. Histopathological and Morphometric Analysis
4.10. Ethical Aspects
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cui, Y.; Song, J.; Cui, C.; Wang, L.; Liang, K.; Wang, C.; Sha, S.; He, Q.; Hu, H.; et al. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem. Biophys. Res. Commun. 2021, 546, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Godea, S.; Ciubotariu, D.; Danciu, M.; Lupușoru, R.V.; Ghiciuc, C.M.; Cernescu, I.; Gheţu, N.; Lupei, M.; Lupușoru, C.E. Improvement in serum lipids and liver morphology after supplementation of the diet with fish oil is more evident under regular feeding conditions than under high-fat or mixed diets in rats. Lipids Health Dis. 2020, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Lima Rocha, J.É.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Silva Primo, M.G.; Cunha Sales, A.L.d.C.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. [Google Scholar] [CrossRef]
- Clevenger, H.C.; Kozimor, A.L.; Paton, C.M.; Cooper, J.A. Acute effect of dietary fatty acid composition on postprandial metabolism in women. Exp. Physiol. 2014, 99, 1182–1190. [Google Scholar] [CrossRef]
- Polley, K.R.; Miller, M.K.; Johnson, M.; Vaughan, R.; Paton, C.M.; Cooper, J.A. Metabolic responses to high-fat diets rich in MUFA v. PUFA. Br. J. Nutr. 2018, 120, 13–22. [Google Scholar] [CrossRef]
- Furtado, M.M.; Rocha, J.L.; Mendes, A.V.d.S.; Neto, R.S.M.; Brito, A.K.d.S.; de Almeida, J.O.C.S.; Queiroz, E.I.R.; França, J.V.d.S.; Sales, A.L.d.C.C.; Vasconcelos, A.G.; et al. Effects of ω-3 PUFA-Rich Oil Supplementation on Cardiovascular Morphology and Aortic Vascular Reactivity of Adult Male Rats Submitted to an Hypercholesterolemic Diet. Biology 2022, 11, 202. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Lin, M.H.; Lu, S.C.; Huang, P.C.; Liu, Y.C.; Liu, S.Y. A high-cholesterol, n-3 polyunsaturated fatty acid diet causes different responses in rats and hamsters. Ann. Nutr. Metab. 2005, 49, 386–391. [Google Scholar] [CrossRef]
- Klingel, S.L.; Metherel, A.H.; Irfan, M.; Rajna, A.; Chabowski, A.; Bazinet, R.P.; Mutch, D.M. EPA and DHA have divergent effects on serum triglycerides and lipogenesis, but similar effects on lipoprotein lipase activity: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1502–1509. [Google Scholar] [CrossRef]
- Innes, J.; Calder, P. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Omega-3 fatty acids: New insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr. Opin. Lipidol. 2013, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) Fatty Acids Susceptible to Peroxidation Are Increased in Plasma and Tissue Lipids of Rats Fed Docosahexaenoic Acid–Containing Oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, M.; Lerotić, I.; Baršić, N.; Tomašić, V.; Jukić, L.V.; Velagić, V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J. Gastroenterol. 2007, 13, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Okada, L.S.d.R.R.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; da Silva, I.D.C.G.; Cordeiro, F.B.; Alves, V.A.F.; Torrinhas, R.S.; Carrilho, F.J.; Puri, P.; et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH-Proteomic and lipidomic insight. Clin. Nutr. 2018, 37, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Lero, M.; Mason, R.P. Are dietary fish oil supplements appropriate for dyslipidemia management? A review of the evidence. Curr. Opin. Lipidol. 2020, 31, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nenseter, M.S.; Drevon, C.A. Dietary polyunsaturates and peroxidation of low density lipoprotein. Curr. Opin. Lipidol. 1996, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Nunzio, M.D.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 121–127. [Google Scholar] [CrossRef]
- Sales, A.L.C.C.; Primo, M.G.S.; Severo, J.S.; Oliveira, A.S.S.S.; Silva, T.M.; Mendes, V.R.; Martins, M.C.C. Effects of omega-3 supplementation on endothelial dysfunction in humans: A systematic review. Rev. Eletrônica Acervo Saúde 2021, 13, e7826. [Google Scholar] [CrossRef]
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, J.; Blair, S.N.; Bonow, R.O.; Brass, L.M.; Fonarow, G.C.; Grundy, S.M.; Hiratzka, L.; Jones, D.; Krumholz, H.M.; et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006, 113, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.; Gagliardi, A.C.M.; Xavier, H.T.; Magnoni, C.D.; Cassani, R.; Lottenberg, A.M.P.; Casella Filho, A.; Araújo, D.B.; Cesena, F.Y.; Alves, R.J.; et al. Sociedade Brasileira de Cardiologia. I Diretriz sobre o consumo de gorduras e saúde cardiovascular [First guidelines on fat consumption and cardiovascular health]. Arq. Bras. Cardiol 2013, 100 (Suppl. S3), 1–40. [Google Scholar] [PubMed]
- De Backer, G.; Ambrosioni, E.; Borch-Johnsen, K.; Brotons, C.; Cifkova, R.; Dallongeville, J.; Ebrahim, S.; Faergeman, O.; Graham, I.; Mancia, G.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2003, 24, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC-Association of Offccial Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Rafacho, A.; Giozzet, V.A.; Boschero, A.C.; Bosqueiro, J.R. Functional alterations in endocrine pancreas of rats with different degrees of dexamethasone-induced insulin resistance. Pancreas 2008, 36, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, R.C.; Prasad, A.S.; Rabbani, P.I.; Cossack, Z.T. Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin. Chem. 1982, 28, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Lavy, R.I.; Fredrickson, D.S. Estimation to density lipoprotein without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Pyles, L.A.; Stejskal, E.; Einzing, S. Spectrophotometric measurement of plasma 2 thiobarbituric acid-reactive substances in the presence of hemoglobin and bilirubin interfernce. Proc. Soc. Exp. Biol. Med. 1993, 202, 407–419. [Google Scholar] [CrossRef]
- Das, K.; Samanta, L.; Chainy, G.B.D. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J. Biochem. Biophys. 2000, 37, 201–204. [Google Scholar]
- Beutler, E. Metabolismo das Células Vermelhas: Um Manual de Métodos Bioquímicos; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]
- Habeeb, A.F.S.A. [37] Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol. 1972, 25, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for isolation and purification of total lipid from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.Y.; O’Doherty, R.M.; Anderson, P.; Lange, A.J.; Newgard, C.B. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J. Biol. Chem. 1998, 273, 31615–31620. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Francisco, P.; Motta, K.; Chagas, T.R.; Dos Santos, C.; Rafacho, A.; Nunes, E.A. Fish oil supplementation attenuates changes in plasma lipids caused by dexamethasone treatment in rats. Applied physiology, nutrition, and metabolism. Appl. Physiol. Nutr. Metab. 2015, 41, 382–390. [Google Scholar] [CrossRef]
- Atkinson, C.; He, S.; Morris, K.; Qiao, F.; Casey, S.; Goddard, M.; Tomlinson, S. Targeted Complement Inhibitors Protect against Posttransplant Cardiac Ischemia and Reperfusion Injury and Reveal an Important Role for the Alternative Pathway of Complement Activation. J. Immunol. 2010, 185, 7007–7013. [Google Scholar] [CrossRef]
- Ansari, M.A.; Iqubal, A.; Ekbbal, R.; Haque, S.E. Effects of nimodipine, vinpocetine and their combination on isoproterenol-induced myocardial infarction in rats. Biomed. Pharmacother. 2019, 109, 1372–1380. [Google Scholar] [CrossRef]
- Brasil. Presidência da República. Law no. 11.794. 8 October 2008. Available online: https://www.planalto.gov.br/ccivil_03/_ato2007-2010/2008/lei/l11794.htm (accessed on 2 January 2024).

| Parameters | G1 Mean ± SEM | G2 Mean ± SEM | G3 Mean ± SEM |
|---|---|---|---|
| Starting weight (g) * | 36.75 ± 0.95 a | 39.00 ± 1.27 a | 44.41 ± 1.84 b |
| Final weight (g) * | 41.62 ± 2.29 a | 29.50 ± 2.10 b | 27.33 ± 2.18 b |
| Total weight gain (g) * | 4.87 ± 2.08 a | −6.75 ± 2.81 b | −14.25 ± 2.01 c |
| Daily food intake (g/day) * | 5.24 ± 0.30 a | 4.13 ± 0.03 b | 4.87± 0.17 a |
| Groups | Hemodynamic Disorders | Parenchyma Disorders | Hyperplasia/Kupffer Cell Hypertrophy | Inflammatory Cell Infiltrates |
|---|---|---|---|---|
| G1 | 1 | 1 | 1 | 1 |
| G2 | 2 | 3 | 2 | 3 |
| G3 | 1 | 1 | 3 | 1 |
| Components | Standard Diet Mean ± SD | Diet HC Mean ± SD | p Value |
|---|---|---|---|
| Carbohydrates (g/100 g) 1 | 40.64 ± 0.83 | 43.63 ± 0.41 | 0.050 |
| Protein (g/100 g) 1 | 39.53 ± 0.98 | 37.51 ± 0.42 | 0.046 * |
| Lipid (g/100 g) 2 | 2.12 ± 0.08 | 3.22 ± 0.09 | 0.000 * |
| Moisture (g/100 g) 2 | 8.47 ± 0.16 | 6.04 ± 0.13 | 0.000 * |
| Ashes (g/100 g) 2 | 9.24 ± 0.29 | 9.60 ± 0.21 | 0.156 |
| VET (kcal/100 g) 2 | 339.79 ± 7.97 | 353.53 ± 4.13 | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, A.L.C.C.; Primo, M.G.S.; Mello Neto, R.S.; Mendes, A.V.S.; Furtado, M.M.; Rocha, J.É.L.; Almeida, J.O.C.S.; França, J.V.S.; Alencar, S.R.; Brito, A.K.S.; et al. A Preliminary Study on Hepatoprotective, Hypolipidemic and Aortic Morphometric Effects of Omega-3-Rich Fish Oil in Hypercholesterolemic Mice. Pharmaceuticals 2024, 17, 72. https://doi.org/10.3390/ph17010072
Sales ALCC, Primo MGS, Mello Neto RS, Mendes AVS, Furtado MM, Rocha JÉL, Almeida JOCS, França JVS, Alencar SR, Brito AKS, et al. A Preliminary Study on Hepatoprotective, Hypolipidemic and Aortic Morphometric Effects of Omega-3-Rich Fish Oil in Hypercholesterolemic Mice. Pharmaceuticals. 2024; 17(1):72. https://doi.org/10.3390/ph17010072
Chicago/Turabian StyleSales, Ana Lina C. C., Maísa G. S. Primo, Renato S. Mello Neto, Ana Victória S. Mendes, Mariely M. Furtado, Joana Érica L. Rocha, José Otávio C. S. Almeida, José Vinícius S. França, Salmon R. Alencar, Ana Karolinne S. Brito, and et al. 2024. "A Preliminary Study on Hepatoprotective, Hypolipidemic and Aortic Morphometric Effects of Omega-3-Rich Fish Oil in Hypercholesterolemic Mice" Pharmaceuticals 17, no. 1: 72. https://doi.org/10.3390/ph17010072






