Solidagenone from Solidago chilensis Meyen Protects against Acute Peritonitis and Lipopolysaccharide-Induced Shock by Regulating NF-κB Signaling Pathway
Abstract
:1. Introduction
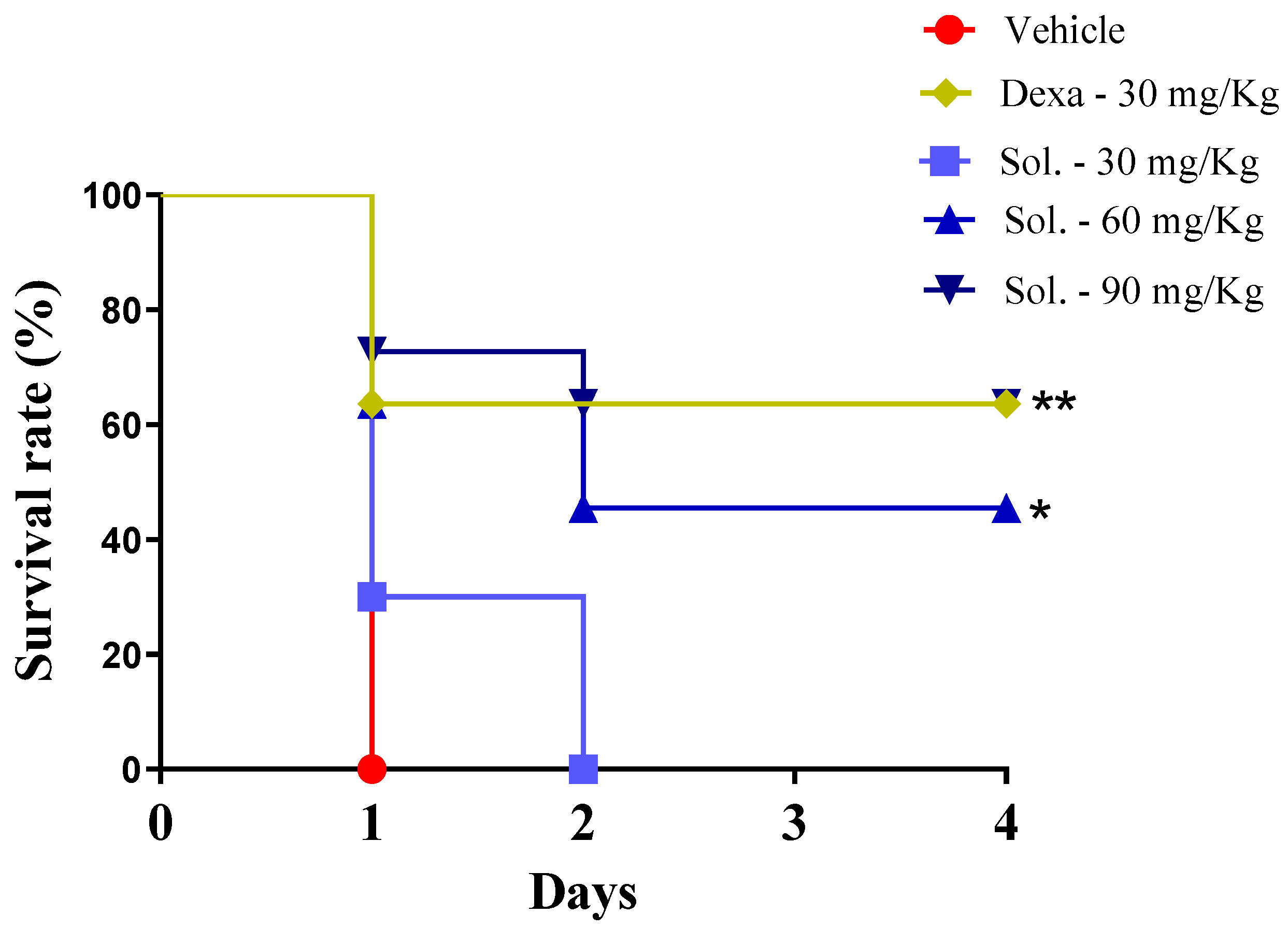
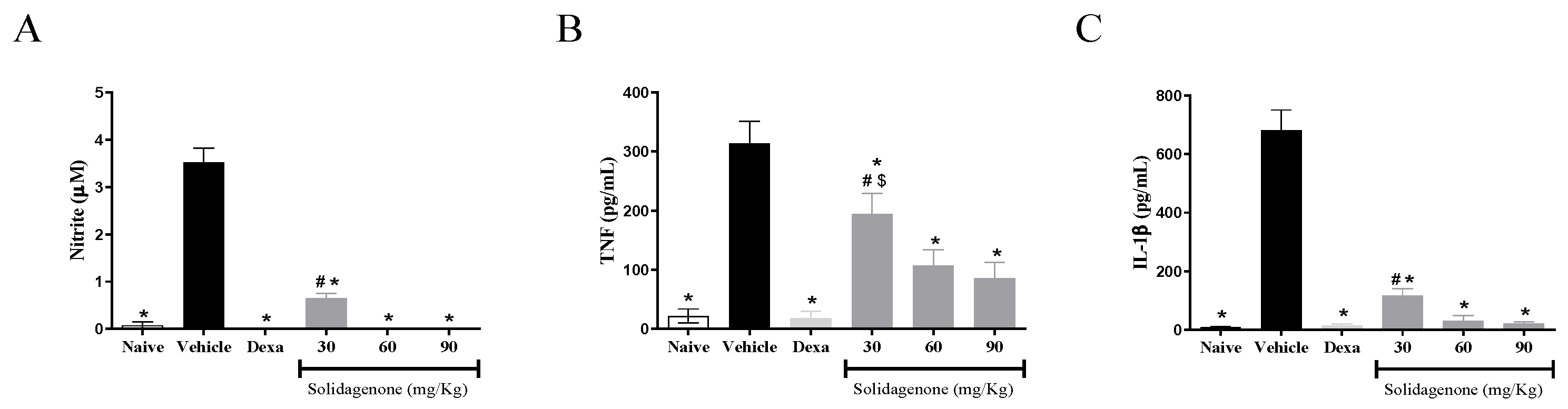
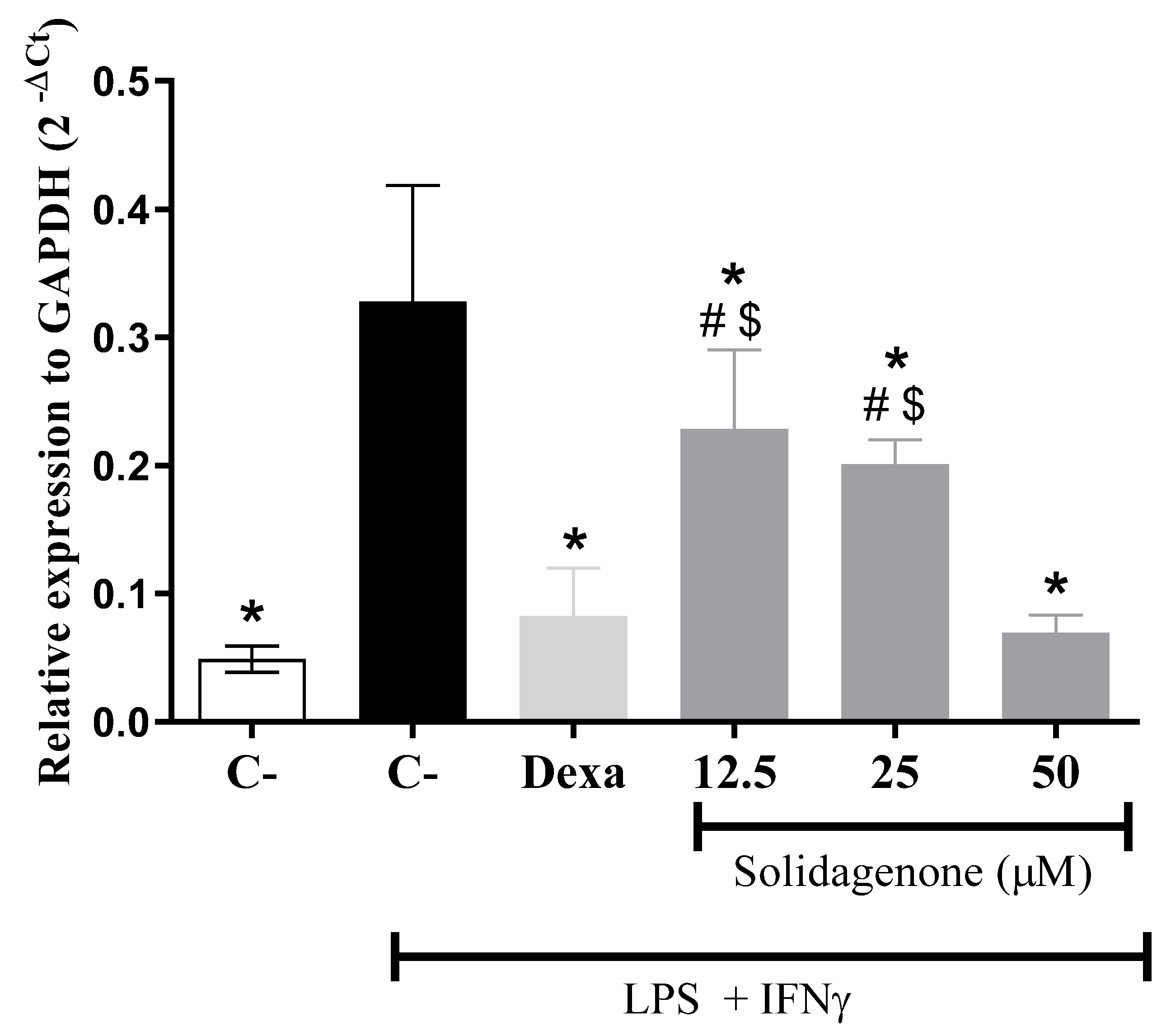
2. Results and Discussion
3. Materials and Methods
3.1. Drugs
3.2. Animals
3.3. Acute Toxicity in Mice
3.4. Induction of Acute Peritonitis in Mice
3.5. LPS-Induced Endotoxin Shock
3.6. Cytokine and Nitric Oxide Production by Resident Macrophages
3.7. Real-Time Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
3.8. Solidagenone Structure
3.9. Crystallographic Protein Structures
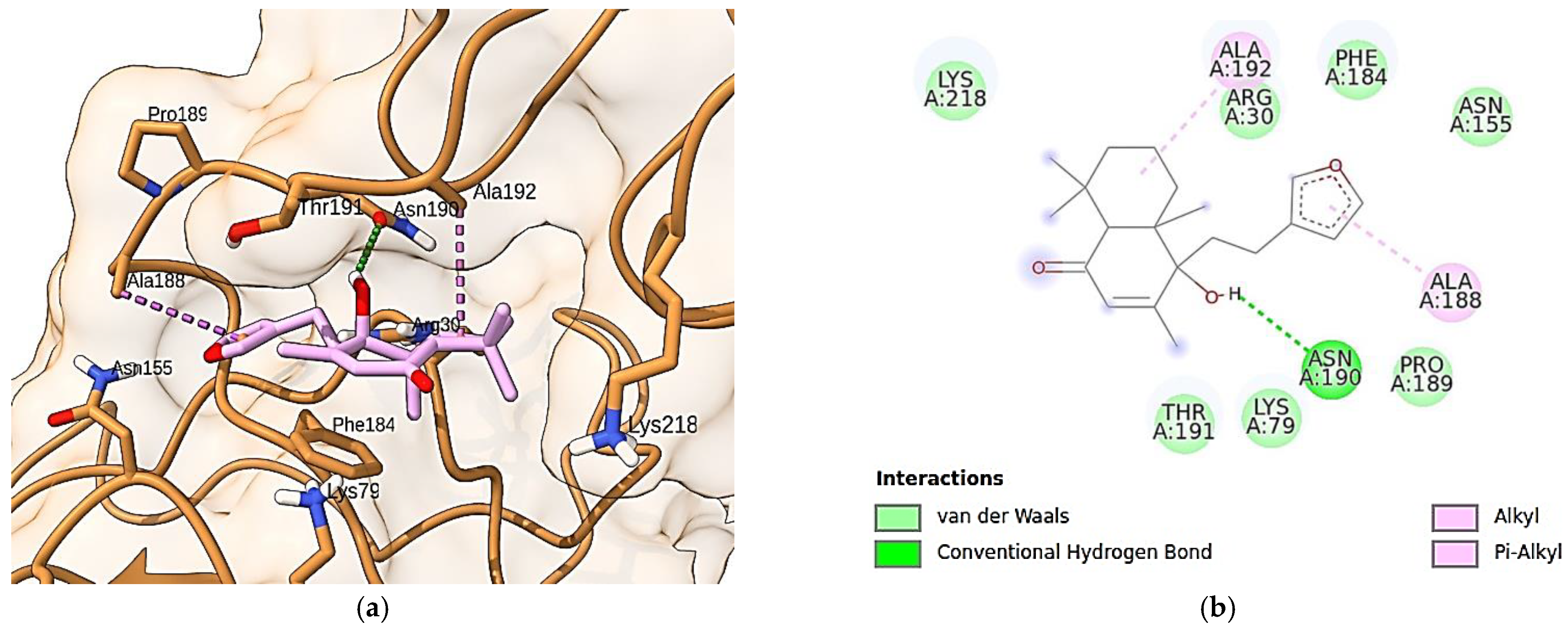
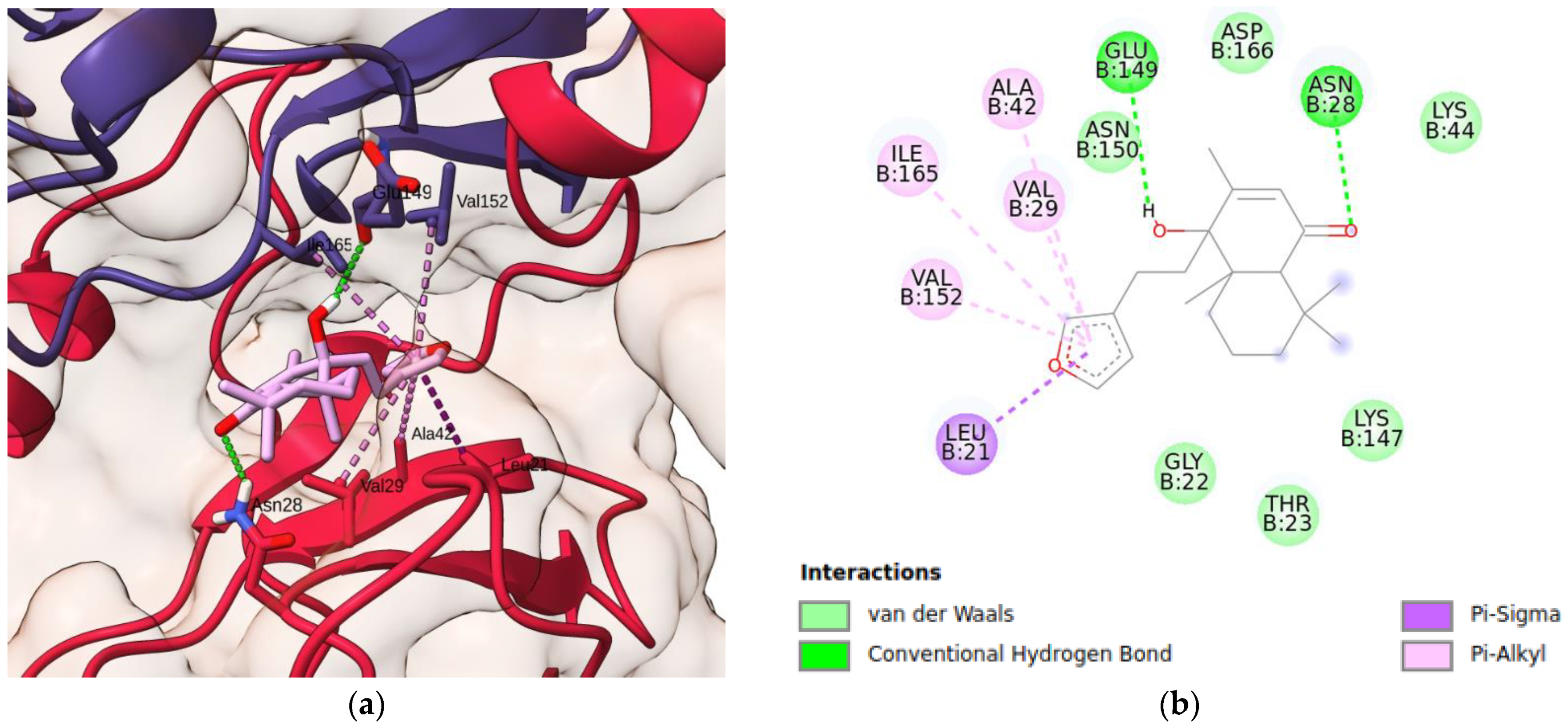
3.10. Molecular Docking Simulations
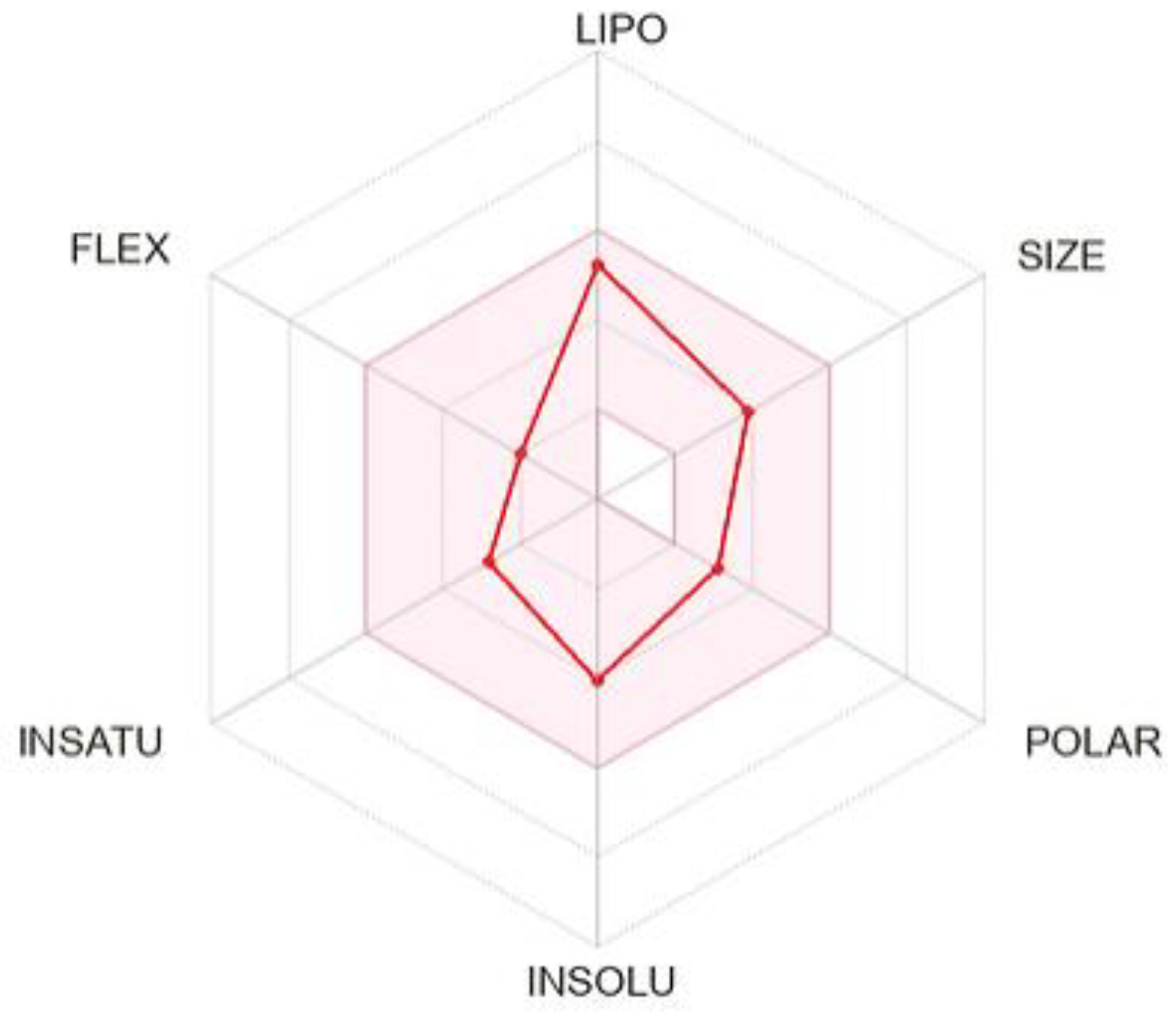
3.11. In Silico ADME
3.12. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathinam, V.A.K.; Chan, F.K.-M. Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef]
- Hawiger, J.; Zienkiewicz, J. Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 2019, 90, e12812. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Bruscoli, S.; Riccardi, C. Defining the role of glucocorticoids in inflammation. Clin. Sci. 2018, 132, 1529–1543. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Mors, W.; Rizzini, C.; Pereira, N. Medicinal Plants of Brazil; Reference Publications, Inc.: Algonac, MI, USA, 2000. [Google Scholar]
- Catarino, H.R.C.; de Godoy, N.P.; Scharlack, N.K.; Neves, L.M.G.; de Gaspi, F.O.; Esquisatto, M.A.M.; do Amaral, M.E.C.; Mendonça, F.A.S.; dos Santos, G.M.T. InGaP 670-nm laser therapy combined with a hydroalcoholic extract of Solidago chilensis Meyen in burn injuries. Lasers Med. Sci. 2015, 30, 1069–1079. [Google Scholar] [CrossRef]
- De Barros, M.; Mota da Silva, L.; Boeing, T.; Somensi, L.B.; Cury, B.J.; de Moura Burci, L.; Santin, J.R.; de Andrade, S.F.; Monache, F.D.; Cechinel-Filho, V. Pharmacological reports about gastroprotective effects of methanolic extract from leaves of Solidago chilensis (Brazilian arnica) and its components quercitrin and afzelin in rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Locateli, G.; de Oliveira Alves, B.; Miorando, D.; Ernetti, J.; Alievi, K.; Zilli, G.A.L.; Serpa, P.Z.; Vecchia, C.A.D.; Mota da Silva, L.; Müller, L.G.; et al. Antidepressant-like effects of solidagenone on mice with bacterial lipopolysaccharide (LPS)-induced depression. Behav. Brain Res. 2020, 395, 112863. [Google Scholar] [CrossRef]
- Valverde, S.S.; Oliveira, T.B.; Souza, S.P. Solidago chilensis Meyen (Asteraceae). Rev. Fitos 2012, 7, 131–136. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodriguez, J.; Astudillo, L. Gastroprotective activity of the diterpene solidagenone and its derivatives on experimentally induced gastric lesions in mice. J. Ethnopharmacol. 2002, 81, 111–115. [Google Scholar] [CrossRef]
- Valverde, S.S.; Santos, B.C.S.; de Oliveira, T.B.; Gonçalves, G.C.; de Sousa, O.V. Solidagenone from Solidago chilensis Meyen inhibits skin inflammation in experimental models. Basic Clin. Pharmacol. Toxicol. 2021, 128, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.F.; Santos, I.P.; de Oliveira, T.B.; Kelly, A.M.; do Reis, B.P.Z.C.; Orge, I.D.; Meira, C.S.; Valverde, S.S.; Soares, M.B.P. The protective effect of solidagenone from Solidago chilensis Meyen in a mouse model of airway inflammation. Basic Clin. Pharmacol. Toxicol. 2022, 130, 44–55. [Google Scholar] [CrossRef]
- Dashti, A.; Shokrzadeh, M.; Karami, M.; Habibi, E. Phytochemical identification, acute and subchronic oral toxicity assessments of hydroalcoholic extract of Acroptilon repens in BALB/c mice: A toxicological and mechanistic study. Heliyon 2022, 8, e08940. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.A.; Bustamante, C.; Astudillo, L.; Schmeda-Hirschmann, G. Gastroprotective activity of solidagenone on experimentally-induced gastric lesions in rats. J. Pharm. Pharmacol. 2002, 54, 399–404. [Google Scholar] [CrossRef]
- Ugwah-Oguejiofor, C.J.; Okoli, C.O.; Ugwah, M.O.; Umaru, M.L.; Ogbulie, C.S.; Mshelia, H.E.; Umar, M.; Njan, A.A. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon 2019, 5, e01179. [Google Scholar] [CrossRef]
- Liz, R.; Vigil, S.V.G.; Goulart, S.; Izabel, M.; Moritz, G.; Schenkel, E.P.; Fröde, T.S. The anti-inflammatory modulatory role of Solidago chilensis Meyen in the murine model of the air pouch. J. Pharm. Pharmacol. 2008, 60, 515–521. [Google Scholar] [CrossRef]
- Goulart, S.; Moritz, M.I.G.; Lang, K.L.; Liz, R.; Schenkel, E.P.; Fröde, T.S. Anti-inflammatory evaluation of Solidago chilensis Meyen in a murine model of pleurisy. J. Ethnopharmacol. 2007, 113, 346–353. [Google Scholar] [CrossRef]
- Bartuzi, P.; Hofker, M.H.; van de Sluis, B. Tuning NF-κB activity: A touch of COMMD proteins. Biochim. Biophys. Acta. 2013, 1832, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lo, Y.-C.; Li, Q.; Napolitano, G.; Wu, X.; Jiang, X.; Dreano, M.; Karin, M.; Wu, H. Crystal structure of inhibitor of ƙB kinase β (IKKβ). Nature 2011, 472, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Scheidereit, C. IκB kinase complexes: Gateways to NF-κB activation and transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Wilde, G.; Notebaert, S.; Vanden Berghe, W.; Haegeman, G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem. Pharmacol. 2002, 64, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, X.-D.; Lamb, A.; Chen, L.-F. Posttranslational modifications of NF-κB: Another layer of regulation for NF-κB signaling pathway. Cell Signal. 2010, 22, 1282–1290. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Bacher, S.; Kracht, M. IκB-independent control of NF-κB activity by modulatory phosphorylations. Trends Biochem. Sci. 2001, 26, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baldwin, A.S., Jr. Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 1998, 273, 29411–29416. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, B.; Miyagi, M.; She, Y.; Gopalan, B.; Huang, D.-B.; Ghosh, G.; Stark, G.R.; Lu, T. PRMT5 dimethylates R30 of the p65 subunit to activate NF-κB. Proc. Natl. Acad. Sci. USA 2013, 110, 13516–13521. [Google Scholar] [CrossRef]
- Meng, X.; Martinez, M.A.; Raymond-Stintz, M.A.; Winter, S.S.; Wilson, B.S. IKK inhibitor bay 11-7082 induces necroptotic cell death in precursor-B acute lymphoblastic leukaemic blasts. Br. J. Haematol. 2010, 148, 487–490. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, M.H.; Kim, E.; Cho, J.Y. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediat. Inflamm. 2012, 2012, 416036. [Google Scholar] [CrossRef]
- Liu, S.; Misquitta, Y.R.; Olland, A.; Johnson, M.A.; Kelleher, K.S.; Kriz, R.; Lin, L.L.; Stahl, M.; Mosyak, L. Crystal structure of a human IκB kinase β asymmetric dimer. J. Biol. Chem. 2013, 288, 22758–22767. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.S.; Azevedo, S.S.; Tomassini, T.C.B. Utilização de CLAE, como paradigma na obtenção e controle do diterpeno solidagenona a partir de inflorescências de Solidago chilensis Meyen (arnica brasileira). Rev. Bras. Farm. 2009, 90, 196–199. [Google Scholar]
- Daltro, S.R.T.; Santos, I.P.; Barros, P.L.; Moreira, D.R.M.; Tomassini, T.C.B.; Ribeiro, I.M.; Ribeiro-dos-Santos, R.; Meira, C.S.; Soares, M.B.P. In vitro and In Vivo Immunomodulatory Activity of Physalis angulata Concentrated Ethanolic Extract. Planta Medica 2021, 87, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.F.O.; Barbosa-Filho, J.M.; Maia, G.L.d.A.; Guimarães, E.T.; Meira, C.S.; Ribeiro-Dos-Santos, R.; de Carvalho, L.C.P.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Jacobs, M.D.; Harrison, S.C. Structure of an IκBα/NF-κB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA; Dassault Systèmes. Discovery Studio Visualizer, Version 21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]

| Behavior or General Appearance | Observations | |||
|---|---|---|---|---|
| Vehicle | Solidagenone (30 mg/kg) | Solidagenone (60 mg/kg) | Solidagenone (90 mg/kg) | |
| Changes in the eyes | No changes | No changes | No changes | No changes |
| Changes in the fur | No changes | No changes | No changes | No changes |
| Changes in the skin | No changes | No changes | No changes | No changes |
| Coma | Absent | Absent | Absent | Absent |
| Convulsions | Absent | Absent | Absent | Absent |
| Diarrhea | Absent | Absent | Absent | Absent |
| Lethargy | Absent | Absent | Absent | Absent |
| Salivation | Absent | Absent | Absent | Absent |
| Sleep | Usual | Usual | Usual | Usual |
| Tremors | Absent | Absent | Absent | Absent |
| Days | Vehicle | Solidagenone (30 mg/kg) | Solidagenone (60 mg/kg) | Solidagenone (90 mg/kg) |
|---|---|---|---|---|
| 0 | 24.3 (±0.6) | 24.1 (±0.3) | 23.8 (±1.6) | 22.8 (±0.9) |
| 7 | 24.2 (±0.3) | 24.7 (±0.5) | 24.0 (±1.8) | 22.8 (±1.2) |
| 14 | 24.4 (±0.5) | 24.8 (±0.6) | 24.5 (±1.5) | 23.0 (±0.7) |
| Group | Dose (mg/kg) | Leukocytes (103 cells/µL) | Thrombocytes (103 mm3) |
|---|---|---|---|
| Naive | - | 3.8 ± 0.9 * | 449.8 ± 49.2 * |
| Vehicle | - | 1.7 ± 0.2 | 334.5 ± 36.7 |
| Dexamethasone | 30 | 3.8 ± 0.4 * | 337.0 ± 26.8 |
| Solidagenone | 30 | 1.9 ± 0.3 | 365.8 ± 15.2 |
| Solidagenone | 60 | 2.2 ± 0.4 | 399.4 ± 3.4 * |
| Solidagenone | 90 | 2.7 ± 0.5 * | 403.5 ± 40.4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.P.; Silva, L.P.; Silva, D.K.C.; dos Reis, B.P.Z.C.; de Oliveira, T.B.; Kelly, A.M.; dos Santos Rodrigues, E.; de Souza, C.V.C.; Oliveira-Costa, J.F.; Valverde, S.S.; et al. Solidagenone from Solidago chilensis Meyen Protects against Acute Peritonitis and Lipopolysaccharide-Induced Shock by Regulating NF-κB Signaling Pathway. Pharmaceuticals 2024, 17, 273. https://doi.org/10.3390/ph17030273
Santos IP, Silva LP, Silva DKC, dos Reis BPZC, de Oliveira TB, Kelly AM, dos Santos Rodrigues E, de Souza CVC, Oliveira-Costa JF, Valverde SS, et al. Solidagenone from Solidago chilensis Meyen Protects against Acute Peritonitis and Lipopolysaccharide-Induced Shock by Regulating NF-κB Signaling Pathway. Pharmaceuticals. 2024; 17(3):273. https://doi.org/10.3390/ph17030273
Chicago/Turabian StyleSantos, Ivanilson Pimenta, Laís Peres Silva, Dahara Keyse Carvalho Silva, Bruna Padilha Zurita Claro dos Reis, Temistocles Barroso de Oliveira, Andressa Maia Kelly, Edivaldo dos Santos Rodrigues, Claudia Valeria Campos de Souza, José Fernando Oliveira-Costa, Simone Sacramento Valverde, and et al. 2024. "Solidagenone from Solidago chilensis Meyen Protects against Acute Peritonitis and Lipopolysaccharide-Induced Shock by Regulating NF-κB Signaling Pathway" Pharmaceuticals 17, no. 3: 273. https://doi.org/10.3390/ph17030273





