Emerging Families of Ion Channels Involved in Urinary Bladder Nociception
Abstract
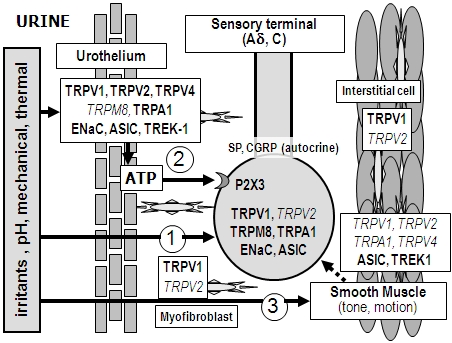
:1. Introduction
2. Peripheral Sensory Machinery in the Urinary Bladder


2.1. Sensory Nerve Endings

2.2. Urothelium
2.3. Detrusor Smooth Muscle and Interstitial Cells of Cajal

3. Nociceptive Ion Channels in the Urinary Bladder
3.1. Degenerin/Epithelial Na+ Channel (Deg/ENaC) Family
3.1.1. Epithelial Na+ Channel (ENaC)
3.1.2. Acid-Sensing Ion Channel (ASIC)
3.2. TRP Channel Family
3.2.1. TRPV1
3.2.2. TRPM8
3.2.3. TRPA1
3.2.4. TRPV4
3.2.5. TRPV2
3.3. Potassium Channels
4. Conclusions
Acknowledgements
References
- de Groat, W.C. Integrative control of the lower urinary tract: preclinical perspective. Br. J. Pharmacol. 2006, 147, S25–S40. [Google Scholar]
- Yoshimura, N.; Seki, S.; Chancellor, M.B.; de Groat, W.C.; Ueda, T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 2002, 59, 61–67. [Google Scholar]
- Hanno, P.M.; Sant, G.R. Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association scientific conference on interstitial cystitis. Urology 2001, 57, 2–6. [Google Scholar]
- Woolf, C.J.; Ma, Q. Nociceptors-noxious stimulus detectors. Neuron 2007, 55, 353–364. [Google Scholar]
- Yoshimura, N.; Kaiho, Y.; Miyazato, M.; Yunoki, T.; Tai, C.; Chancellor, M.B.; Tyagi, P. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 377, 437–448. [Google Scholar]
- Araki, I.; Du, S.; Kobayashi, H.; Sawada, F.; Mochizuki, T.; Zakoji, H.; Takeda, M. Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int. J. Urol. 2008, 15, 681–687. [Google Scholar]
- Voets, T.; Talavera, K.; Owsianik, G.; Nilius, B. Sensing with TRP channels. Nat. Chem. Biol. 2005, 1, 85–92. [Google Scholar] [Green Version]
- Kellenberger, S.; Schild, L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002, 82, 735–767. [Google Scholar]
- Chancellor, M.B.; de Groat, W.C. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J. Urol. 1999, 162, 3–11. [Google Scholar]
- Payne, C.K.; Mosbaugh, P.G.; Forrest, J.B.; Evans, R.J.; Whitmore, K.E.; Antoci, J.P.; Perez-Marrero, R.; Jacoby, K.; Diokno, A.C.; O'Reilly, K.J.; Griebling, T.L.; Vasavada, S.P.; Yu, A.S.; Frumkin, L.R. ICOS RTX Study Group. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J. Urol. 2005, 173, 1590–1594. [Google Scholar] [PubMed]
- Burnstock, G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001, 22, 182–188. [Google Scholar]
- Tsunozaki, M.; Bautista, D.M. Mammalian somatosensory mechanotransduction. Curr. Opin. Neurobiol. 2009, 19, 362–369. [Google Scholar]
- Häbler, H.J.; Jänig, W.; Koltzenburg, M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol. 1990, 425, 545–562. [Google Scholar]
- Häbler, H.J.; Jänig, W.; Koltzenburg, M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J. Physiol. 1993, 463, 449–460. [Google Scholar]
- Shea, V.K.; Cai, R.; Crepps, B.; Mason, J.L.; Perl, E.R. Sensory fibers of pelvic nerve innervating the rat’s urinary bladder. J. Neurophysiol. 2000, 84, 1924–1933. [Google Scholar]
- Gabella, G.; Davis, C. Distribution of afferent axons in the bladder of rats. J. Neurocytol. 1998, 27, 141–155. [Google Scholar]
- Xu, L.; Gebhart, G.F. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J. Neurophysiol. 2008, 99, 244–253. [Google Scholar]
- Zagorodnyuk, V.P.; Gibbins, I.L.; Costa, M.; Brookes, S.J.H.; Gregory, S.J. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J. Physiol. 2007, 585, 147–163. [Google Scholar] [PubMed]
- Zagorodnyuk, V.P.; Brookes, S.J.H.; Spencer, N.J.; Gregory, S. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J. Physiol. 2009, 587, 3523–3538. [Google Scholar]
- Yu, Y.; de Groat, W.C. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am. J. Physiol. Renal. Physiol. 2008, 294, F1146–F1156. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Gilpin, S.A.; Dixon, J.S.; Richmond, D.H.; Sutherst, J.R. Increase in presumptive sensory nerves of the urinary bladder in idiopathic detrusor instability. Brit. J. Urol. 1992, 70, 370–372. [Google Scholar]
- Welsh, M.J.; Price, M.P.; Xie, J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J. Biol. Chem. 2002, 277, 2369–2372. [Google Scholar]
- Smet, P.J.; Moore, K.H.; Jonavicius, J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab. Invest. 1997, 77, 37–49. [Google Scholar] [PubMed]
- Patacchini, R.; Santicioli, P.; Giuliani, S.; Maggi, C.A. Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur. J. Pharmacol. 2005, 509, 171–177. [Google Scholar]
- Maggi, C.A.; Meli, A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988, 19, 1–43. [Google Scholar]
- Andersson, K.E. Bladder activation: afferent mechanisms. Urology 2002, 59, 43–50. [Google Scholar]
- Birder, L.A. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am. J. Physiol. Renal. Physiol. 2005, 289, F489–F495. [Google Scholar]
- Sabirov, R.Z.; Okada, Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn. J. Physiol. 2004, 54, 7–14. [Google Scholar]
- Rong, W.; Spyer, K.M.; Burnstock, G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J. Physiol. 2002, 541, 591–600. [Google Scholar]
- Vlaskovska, M.; Kasakov, L.; Rong, W.; Bodin, P.; Bardini, M.; Cockayne, D.A.; Ford, A.P.D.W.; Burnstock, G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 2001, 21, 5670–5677. [Google Scholar]
- Sun, Y.; Keay, S.; De Deyne, P.G.; Chai, T.C. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 2001, 166, 1951–1956. [Google Scholar]
- Birder, L.A.; Barrick, S.R.; Roppolo, J.R.; Kanai, A.J.; de Groat, W.C.; Kiss, S.; Buffington, C.A. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am. J. Physiol. Renal. Physiol. 2003, 285, F423–F429. [Google Scholar]
- Brady, C.M.; Apostolidis, A.; Yiangou, Y.; Baecker, P.A.; Ford, A.P.; Freeman, A.; Jacques, T.S.; Fowler, C.J.; Anand, P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of Intravesical resiniferatoxin. Eur. Urol. 2004, 46, 247–253. [Google Scholar]
- Birder, L.A.; Ruan, H.Z.; Chopra, B.; Xiang, Z.; Barrick, S.; Buffington, C.A.; Roppolo, J.R.; Ford, A.P.D.W.; de Groat, W.C.; Burnstock, G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. Renal. Physiol. 2004, 287, F1084–F1091. [Google Scholar]
- Iijima, K.; De Wachter, S.; Wyndaele, J.J. Effects of the M3 receptor selective muscarinic antagonist darifenacin on bladder afferent activity of the rat pelvic nerve. Eur. Urol. 2007, 52, 842–849. [Google Scholar]
- Mukerji, G.; Yiangou, Y.; Grogono, J.; Underwood, J.; Agarwal, S.K.; Khullar, V.; Anand, P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J. Urol. 2006, 176, 367–373. [Google Scholar]
- Jositsch, G.; Papadakis, T.; Haberberger, R.V.; Wolff, M.; Wess, J.; Kummer, W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 389–395. [Google Scholar]
- Lee, T.; Hedlund, P.; Newgreen, D.; Andersson, K.E. Urodynamic effects of a novel EP1 receptor antagonist in normal rats and rats with bladder outlet obstruction. J. Urol. 2007, 177, 1562–1567. [Google Scholar]
- Jugus, M.J.; Jaworski, J.P.; Patra, P.B.; Jin, J.; Morrow, D.M.; Laping, N.J.; Edwards, R.M.; Thorneloe, K.S. Dual modulation of urinary bladder activity and urine flow by prostanoid EP3 receptors in the conscious rat. Br. J. Pharmacol. 2009, 158, 372–381. [Google Scholar]
- Girard, B.M.; Wolf-Johnston, A.; Braas, K.M.; Birder, L.A.; May, V.; Vizzard, M.A. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J. Mol. Neurosci. 2008, 36, 310–320. [Google Scholar]
- Sun, Y.; MaLossi, J.; Jacobs, S.C.; Chai, T.C. Effect of doxazosin on stretch-activated adenosine triphosphate release in bladder urothelial cells from patients with benign prostatic hyperplasia. Urology 2002, 60, 351–356. [Google Scholar]
- Yoshida, M.; Masunaga, K.; Nagata, T.; Maeda, Y.; Miyamoto, Y.; Kudoh, J.; Homma, Y. Attenuation of non-neuronal adenosine triphosphate release from human bladder mucosa by antimuscarinic agents. LUTS 2009, 1, 88–92. [Google Scholar]
- Andersson, K.E. Detrusor myocyte activity and afferent signaling. Neurourol. Urodyn 2010, 29, 97–106. [Google Scholar]
- McCloskey, K.D. Interstitial cells in the urinary bladder–localization and function. Neurourol. Urodyn 2010, 29, 82–87. [Google Scholar]
- Zagorodnyuk, V.P.; Gregory, S.; Costa, M.; Brookes, S.J.H.; Tramontana, M.; Giuliani, S.; Maggi, C.A. Spontaneous release of acetylcholine from autonomic nerves in the bladder. Br. J. Pharmacol. 2009, 157, 607–619. [Google Scholar]
- McCarthy, C.J.; Zabbarova, I.V.; Brumovsky, P.R.; Gebhart, G.F.; Kanai, A.J. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J. Urol. 2009, 181, 1459–1466. [Google Scholar]
- Mills, L.W.; Greenland, J.E.; McMurray, G.; McCoy, R.; Ho, K.M.T.; Noble, J.G.; Brading, A.F. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J. Urol. 2000, 163, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.J.; Hedlund, P.; Andersson, K.E.; Brading, A.F.; Hussain, I.; Fowler, C.; Landon, D.N. Morphology, phenotype and ultrastructure of fibroblastic cells from normal and neuropathic human detrusor: absence of myofibroblast characteristics. J. Urol. 2003, 169, 1573–1576. [Google Scholar]
- Sanders, K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996, 111, 492–515. [Google Scholar]
- Sergeant, G.P.; Hollywood, M.A.; McCloskey, K.D.; Thornbury, K.D.; McHale, N.G. Specialised pacemaking cells in the rabbit urethra. J. Physiol. 2000, 526, 359–366. [Google Scholar]
- Biers, S.M.; Reynard, J.M.; Doore, T.; Brading, A.F. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2005, 97, 612–616. [Google Scholar]
- Hashitani, H.; Yanai, Y.; Suzuki, H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscle of the guinea-pig urinary bladder. J. Physiol. 2004, 559.2, 567–581. [Google Scholar]
- Fry, C.H.; Sui, G.P.; Kanai, A.J.; Wu, C. The function of suburothelial myofibroblasts in the bladder. Neurourol. Urodyn 2007, 26, 914–919. [Google Scholar]
- Roosen, A.; Datta, S.N.; Chowdhury, R.A.; Patel, P.M.; Kalsi, V.; Elneil, S.; Dasgupta, P.; Kessler, T.M.; Khan, S.; Panicker, J.; Fry, C.H.; Brandner, S.; Fowler, C.J.; Apostolidis, A. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur. Urol. 2009, 55, 1440–1449. [Google Scholar] [PubMed]
- Kopp, U.C.; Matsushita, K.; Sigmund, R.D.; Smith, L.A.; Watanabe, S.; Stokes, J.B. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1780–R1792. [Google Scholar]
- Smith, P.R.; Mackler, S.A.; Weiser, P.C.; Brooker, D.R.; Ahn, Y.J.; Harte, B.J.; McNulty, K.A.; Kleyman, T.R. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am. J. Physiol. Renal. Physiol. 1998, 274, F91–F96. [Google Scholar]
- Araki, I.; Du, S.; Kamiyama, M.; Mikami, Y.; Matsushita, K.; Komuro, M.; Furuya, Y.; Takeda, M. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology 2004, 64, 1255–1260. [Google Scholar]
- Du, S.; Araki, I.; Mikami, Y.; Zakoji, H.; Beppu, M.; Yoshiyama, M.; Takeda, M. Amiloride-sensitive ion channels in urinary bladder epithelium involved in mechanosensory transduction by modulating stretch-evoked adenosine triphosphate release. Urology 2007, 69, 590–595. [Google Scholar]
- Ferguson, D.R.; Kennedy, I.; Burton, T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J. Physiol. 1997, 505, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Price, M.P.; Welsh, M.J. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006, 29, 578–586. [Google Scholar]
- Kobayashi, H.; Yoshiyama, M.; Zakoji, H.; Takeda, M.; Araki, I. Sex differences in expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int. 2009, 104, 1746–1751. [Google Scholar]
- Corrow, K.; Girard, B.M.; Vizzard, M.A. Expression and response of acid-sensing ion channels (ASICs) in urinary bladder to cyclophosphamide (CYP)-induced cystitis. Am. J. Physiol. Renal. Physiol. 2010, 86, 1120–1127. [Google Scholar]
- Sadananda, P.; Shang, F.; Liu, L.; Mansfield, K.J.; Burcher, E. Release of ATP from rat urinary bladder mucosa: role of acid, vanilloids and stretch. Br. J. Pharmacol. 2009, 158, 1655–1662. [Google Scholar]
- Kullmann, F.A.; Shah, M.A.; Birder, L.A.; de Groat, W.C. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am. J. Physiol. Renal. Physiol. 2009, 296, F892–F901. [Google Scholar]
- Page, A.J.; Brierley, S.M.; Martin, C.M.; Price, M.; Symonds, E.; Butler, R.; Wemmie, J.A.; Blackshaw, L.A. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 2005, 54, 1408–1415. [Google Scholar] [PubMed]
- Jones III, R.C.W.; Xu, L.; Gebhart, G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar]
- Everaerts, W.; Gevaert, T.; Nilius, B.; De Ridder, D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol. Urodyn 2008, 27, 264–273. [Google Scholar] [Green Version]
- Christensen, A.D.; Corey, D.P. TRP channels in mechanosensation: direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.; Cruz, F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 373, 287–299. [Google Scholar]
- Lazzeri, M.; Vannucchi, M.G.; Zardo, C.; Spinelli, M.; Beneforti, P.; Turini, D.; Faussone-Pellegrini, M.S. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur. Urol. 2004, 46, 792–798. [Google Scholar]
- Everaerts, W.; Sepulvera, M.R.; Gavaert, T.; Roskams, T.; Nillius, B.; De Ridder, D. Where is TRPV1 expressed in the bladder, do see the real channel? Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 421–425. [Google Scholar]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; de Groat, W.C.; Apodaca, G.; Watkins, S.; Caterina, M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar]
- Yoshiyama, M.; Araki, I.; Kobayashi, H.; Zakoji, H.; Takeda, M. Functional roles of TRPV1 channels in lower urinary tract irritated by acetic acid: in vivo evaluations on the sex difference in decerebrate unanesthetized mice. Am. J. Physiol. Renal. Physiol. 2010, 298, F1351–F1359. [Google Scholar]
- Zeihofer, H.U.; Kress, M.; Swandulla, D. Fractional Ca2+ currents through capsaicin- and proton-activated ion channels in rat dorsal root ganglion neurons. J. Physiol. 1997, 503, 67–78. [Google Scholar]
- Rigoni, M.; Trevisani, M.; Gazzieri, D.; Nadaletto, R.; Tognetto, M.; Creminon, C.; Davis, J.B.; Campi, B.; Amadesi, S.; Geppetti, P.; Harrison, S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br. J. Pharmacol. 2003, 138, 977–985. [Google Scholar]
- Dinis, P.; Charrus, A.; Avelino, A.; Yaqoob, M.; Bevan, S.; Nagy, I.; Cruz, F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J. Neurosci. 2004, 24, 11253–11263. [Google Scholar]
- Wang, Z.Y.; Wang, P.; Merriam, F.V.; Bjorling, D.E. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 2008, 139, 158–167. [Google Scholar]
- Charrua, A.; Cruz, C.D.; Narayanan, S.; Gharat, L.; Gullapalli, S.; Cruz, F.; Avelino, A. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J. Urol. 2009, 181, 379–386. [Google Scholar] [PubMed]
- Daly, D.; Rong, W.; Chess-Williams, R.; Chapple, C.; Grundy, D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J. Physiol. 2007, 583.2, 663–674. [Google Scholar]
- Charrua, A.; Reguenga, C.; Cordeiro, J.M.; Correiade-Sa, P.; Paule, C.; Nagy, I.; Cruz, F.; Avelino, A. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J. Urol. 2009, 182, 2944–2950. [Google Scholar]
- Yamada, T.; Ugawa, S.; Ueda, T.; Ishida, Y.; Kajita, K.; Shimada, S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J. Histochem. Cytochem. 2009, 57, 277–287. [Google Scholar]
- Xu, X.; Gordon, E.; Lin, Z.; Lozinskaya, I.M.; Chen, Y.; Thorneloe, K.S. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels 2009, 3, 156–160. [Google Scholar]
- Everaerts, W.; Vriens, J.; Owsianik, G.; Appendino, G.; Voets, T.; De Ridder, D.; Nilius, B. Functional characterisation of transient receptor potential channels in mouse urothelial cells. Am. J. Physiol. Renal. Physiol. 2010, 298, F692–F701. [Google Scholar]
- Mukerji, G.; Yiangou, Y.; Corcoran, S.L.; Selmer, I.S.; Smith, G.D.; Benham, C.D.; Bountra, C.; Agarwal, S.K.; Anand, P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006, 6, 6. [Google Scholar]
- Nomoto, Y.; Yoshida, A.; Ikeda, S.; Kamikawa, Y.; Harada, K.; Ohwatashi, A.; Kawahira, K. Effect of menthol on detrusor smooth-muscle contraction and micturition reflex in rats. Urology 2008, 72, 701–705. [Google Scholar]
- Lashinger, E.S.R.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Renal. Physiol. 2008, 295, F803–F810. [Google Scholar]
- Hayashi, T.; Kondo, T.; Ishimatsu, M.; Yamada, S.; Nakamura, K.; Matsuoka, K.; Akasu, T. Expression of the TRPM8-immunoreactivity in dorsal root ganglion neurons innervating the rat urinary bladder. Neurosci. Res. 2009, 65, 245–251. [Google Scholar]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar]
- Mahieu, F.; Owsianik, G.; Verbert, L.; Janssens, A.; De Smedt, H.; Nillius, B.; Voets, T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J. Biol. Chem. 2007, 282, 3325–3336. [Google Scholar]
- Du, S.; Araki, I.; Kobayashi, H.; Zakoji, H.; Sawada, N.; Takeda, M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology 2008, 72, 450–455. [Google Scholar] [PubMed]
- Nagata, K.; Duggan, A.; Kumar, G.; Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar]
- Du, S.; Araki, I.; Yoshiyama, M.; Nomura, T.; Takeda, M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology 2007, 70, 826–831. [Google Scholar]
- Streng, T.; Axelsson, H.E.; Hedlund, P.; Andersson, D.A.; Jordt, S.E.; Bevan, S.; Andersson, K.E.; Hogestatt, E.D.; Zygmunt, P.M. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channels in rat urinary bladder. Eur. Urol. 2008, 53, 391–400. [Google Scholar]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; McIntyre, P.; Jegla, T.; Bevan, S.; Patapoutian, A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar]
- Andrade, E.L.; Ferreira, J.; Andre, E.; Calixto, J.B. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem. Pharmacol. 2006, 72, 104–114. [Google Scholar]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trp4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar]
- Gavaert, T.; Vriens, J.; Segal, A.; Everaerts, W.; Roskams, T.; Talavera, K.; Owsianik, G.; Liedtke, W.; Daelemans, D.; Dewachter, I.; Van Leuven, F.; Voets, T.; De Ridder, D.; Nilius, B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 2007, 117, 3453–3462. [Google Scholar]
- Birder, L.; Kullmann, F.A.; Lee, H.; Barrick, S.; de Groat, W.; Kanai, A.; Caterina, M. Activation of urothelial transient receptor potential vanilloid 4 by 4αphorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J. Pharmacol. Exp. Ther. 2007, 323, 227–235. [Google Scholar]
- Mochizuki, T.; Sokabe, T.; Araki, I.; Fujishita, K.; Shibasaki, K.; Uchida, K.; Koizumi, S.; Takeda, M.; Tominaga, M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J. Biol. Chem. 2009, 284, 21257–21264. [Google Scholar]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.R.; Gordon, E.; Evans, L.; Misajet, B.A.; DeMarini, D.J.; Nation, J.H.; Casillas, L.N.; Marquis, R.W.; Votta, B.J.; Sheardown, S.A.; Xu, X.; Brooks, D.P.; Laping, N.J.; Westfall, T.D. N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide(GSK10-16790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar]
- Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Kiss, S.; Nealen, M.L.; Burke, N.E.; Dineley, K.E.; Watkins, A.; Reynolds, I.J.; Caterina, M.J. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13396–13401. [Google Scholar]
- Ost, D.; Roskams, T.; Van Der Aa, F.; De Ridder, D. Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. J. Urol. 2002, 168, 293–297. [Google Scholar]
- Caprodossi, S.; Lucciarini, R.; Amantini, C.; Nabissi, M.; Canesin, G.; Ballarini, P.; Di Spilimbergo, A.; Cardarelli, M.A.; Servi, L.; Mammana, G.; Santoni, G. Transient receptor potential vanilloid type 2 (TRPV2) expression in normal urothelium and in urothelial carcinoma of human bladder: correlation with the pathologic stage. Eur. Urol. 2008, 54, 612–620. [Google Scholar]
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: physiology and pathphysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar]
- Darblade, B.; Behr-Roussel, D.; Oger, S.; Hieble, J.-P.; Lebret, T.; Gorny, D.; Benoit, G.; Alexandre, L.; Giuliano, F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 2006, 68, 442–448. [Google Scholar]
- Yu, Y.; de Groat, W.C. Effects of ZD6169, a K(ATP) channel opener, on the micturition reflex in the rat. J. Pharmacol. Exp. Ther. 1999, 290, 825–831. [Google Scholar] [PubMed]
- Wojdan, A.; Freeden, C.; Woods, M.; Oshiro, G.; Spinelli, W.; Colatsky, T.J.; Sheldon, J.H.; Norton, N.W.; Warga, D.; Antane, M.M.; Antane, S.A.; Butera, J.A.; Argentieri, T.M. Comparison of the potassium channel openers, WAY-133537, ZD6169, and celikalim on isolated bladder tissue and in vivo bladder instability in rat. J. Pharmacol. Exp. Ther. 1999, 289, 1410–1418. [Google Scholar] [PubMed]
- Tanaka, M.; Sasaki, Y.; Kimura, Y.; Fukui, T.; Hamada, K.; Ukai, Y. A novel pyrrole derivative, NS-8, suppresses the rat micturition reflex by inhibiting afferent pelvic nerve activity. BJU Int. 2003, 92, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zakoji, H.; Araki, I.; Beppu, M.; Yoshiyama, M.; Kobayashi, H.; Du, S.; Sawada, N.; Mochizuki, T.; Fukasawa, M.; Takihana, Y.; Takeda, M. The expression and role of large conductance, voltage- and Ca2+-activated K+ (BK) channels in the urinary bladder: the alteration of subunit expression profile in association with bladder outlet obstruction, and the effect of BK channel on afferent pathway in lower urinary tract. J. Urol. 2007, 177, 325. [Google Scholar] [PubMed]
- Tang, Q.Y.; Qi, Z.; Naruse, K.; Sokabe, M. Characterization of a functionally expressed stretch-activated BKCa channel cloned from chick ventricular myocytes. J. Membr. Biol. 2003, 96, 185–200. [Google Scholar]
- Sanders, K.M.; Koh, S.D. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J. Physiol. 2006, 570, 37–43. [Google Scholar]
- Tertyshnikova, S.; Knox, R.J.; Plym, M.J.; Thalody, G.; Griffin, C.; Neelands, T.; Harden, D.G.; Signor, L.; Weaver, D.; Myers, R.A.; Lodge, N.J. BL-1249[(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: a putative potassium channel opener with bladder-relaxant properties. J. Pharmacol. Exp. Ther. 2005, 313, 250–259. [Google Scholar]
- Baker, S.A.; Hatton, W.J.; Han, J.; Hennig, G.W.; Britton, F.C.; Koh, S.D. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J. Urol. 2010, 183, 793–800. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Araki, I.; Yoshiyama, M.; Kobayashi, H.; Mochizuki, T.; Du, S.; Okada, Y.; Takeda, M. Emerging Families of Ion Channels Involved in Urinary Bladder Nociception. Pharmaceuticals 2010, 3, 2248-2267. https://doi.org/10.3390/ph3072248
Araki I, Yoshiyama M, Kobayashi H, Mochizuki T, Du S, Okada Y, Takeda M. Emerging Families of Ion Channels Involved in Urinary Bladder Nociception. Pharmaceuticals. 2010; 3(7):2248-2267. https://doi.org/10.3390/ph3072248
Chicago/Turabian StyleAraki, Isao, Mitsuharu Yoshiyama, Hideki Kobayashi, Tsutomu Mochizuki, Shuqi Du, Yusaku Okada, and Masayuki Takeda. 2010. "Emerging Families of Ion Channels Involved in Urinary Bladder Nociception" Pharmaceuticals 3, no. 7: 2248-2267. https://doi.org/10.3390/ph3072248
APA StyleAraki, I., Yoshiyama, M., Kobayashi, H., Mochizuki, T., Du, S., Okada, Y., & Takeda, M. (2010). Emerging Families of Ion Channels Involved in Urinary Bladder Nociception. Pharmaceuticals, 3(7), 2248-2267. https://doi.org/10.3390/ph3072248