Abstract
Terbium-149 is among the most interesting therapeutic nuclides for medical applications. It decays by emission of short-range α-particles (Eα = 3.967 MeV) with a half-life of 4.12 h. The goal of this study was to investigate the anticancer efficacy of a 149Tb-labeled DOTA-folate conjugate (cm09) using folate receptor (FR)-positive cancer cells in vitro and in tumor-bearing mice. 149Tb was produced at the ISOLDE facility at CERN. Radiolabeling of cm09 with purified 149Tb resulted in a specific activity of ~1.2 MBq/nmol. In vitro assays performed with 149Tb-cm09 revealed a reduced KB cell viability in a FR-specific and activity concentration-dependent manner. Tumor-bearing mice were injected with saline only (group A) or with 149Tb-cm09 (group B: 2.2 MBq; group C: 3.0 MBq). A significant tumor growth delay was found in treated animals resulting in an increased average survival time of mice which received 149Tb-cm09 (B: 30.5 d; C: 43 d) compared to untreated controls (A: 21 d). Analysis of blood parameters revealed no signs of acute toxicity to the kidneys or liver in treated mice over the time of investigation. These results demonstrated the potential of folate-based α-radionuclide therapy in tumor-bearing mice.
1. Introduction
Targeted radionuclide therapy using β−-particle-emitting radionuclides (e.g., 131I, 90Y, 177Lu) of variable energies is employed in clinical routine. For this purpose a variety of tumor-targeted biomolecules have been used, ranging from small-molecular weight compounds (e.g., 131I-MIBG), to peptides (e.g., 177Lu-DOTATATE) and monoclonal antibodies (90Y-Ibritumomab, Zevalin®) [1,2]. A promising option for a potential improvement of the therapeutic efficacy of radioendotherapy may be the selection of appropriate radionuclides. α-Particles of medically interesting radionuclides provide a 200- to 1,000-fold higher linear energy transfer (LET) than β−-particles [3,4]. Therefore, and because of the much shorter path-length (25–100 μm) of α-particles compared to β−-particles (0.05–12 mm), α-particle-emitting nuclides may be interesting for targeted radionuclide therapy of micrometastases or even single cancer cells.
Allen et al. suggested the radiolanthanide 149Tb as the preferred radionuclide for α-radionuclide therapy [3]. A decay scheme with a list of significant emitted radiation of 149Tb and its daughter nuclides has been previously published by Beyer et al. [5]. 149Tb decays with a half-life of 4.12 h by emission of short-range α-particles (Eα = 3.967 MeV, I = 16.7%) [4,5]. In addition, it emits γ-rays of an energy (Eγ = 165 keV, 26.4%) potentially suitable for single photon emission computed tomography (SPECT) and positrons (Eβ+average = 638 keV, 3.8%) which may be detected via positron emission tomography (PET) (Figure 1A) [4,6]. However, there also exists suitable diagnostic matched nuclides such as 155Tb (T1/2 = 5.32 d, Eγ= 87 keV, I = 32% and 105 keV, I = 25%) and 152Tb (T1/2 = 17.5 h, Eβ+average = 1.08 MeV, I = 17%) for imaging purposes via SPECT and PET, respectively [7]. Tb can be stably coordinated by macrocyclic chelators (e.g., DOTA) as it has been recently demonstrated using the β−-emitting nuclide 161Tb [7,8,9]. 149Tb is among the most attractive candidates of α-emitting radiometals for targeted radionuclide therapy. Other α-emitters have either a very short half-life (213Bi: T1/2 = 46 min, 212Bi: T1/2 = 61 min) or a complicated decay cascade of 4 to 5 α-emissions (225Ac: T1/2 = 10.0 d, 227Th: T1/2 = 18.7 d) [4,10,11]. The longer-lived in vivo generator 212Pb/212Bi (T1/2 = 10.64 h) might be a more favorable solution but could suffer from release of the 212Bi from the DOTA complex [12]. In such cases the decay of the daughter nuclides may occur in non-targeted organs which could cause undesired toxicity to healthy tissue [13]. In spite of the fact that the α-emitting 211At (T1/2 = 7.21 h) is considered appropriate for medical use with regard to the physical properties, a major impediment to practical applications is the low in vivo stability of astatine bonds with aromatic carbon bonds [14]. However, current availability of 149Tb is poor due to production routes which are not easily accessible [6]. Potential production routes include irradiation of rare 152Gd targets with high energy protons (>50 MeV) or the use of light ions as projectiles of >500 MeV protons for spallation reactions. However, in both cases mass separation is required to avoid radioisotopic impurities.
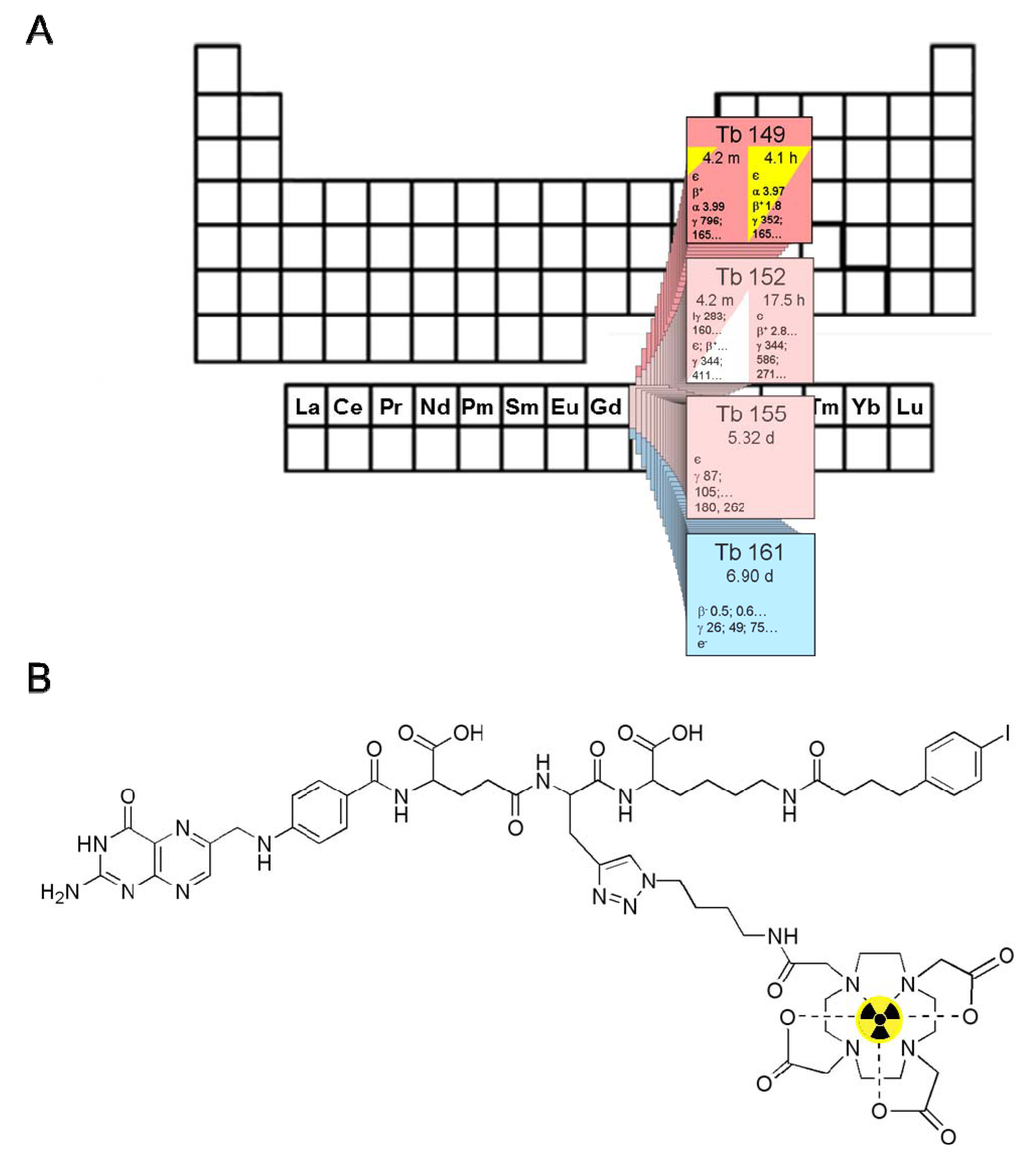
Figure 1.
(A) The α-particle-emitting 149Tb as one of four medically interesting terbium-nuclides which belong to the series of chemical elements called lanthanides (15 elements from La to Lu); (B) Chemical structure of the radiolabeled DOTA-folate conjugate (cm09) with an albumin binding entity (speculative coordination sphere of the Tb-DOTA-complex).
The aim of the present study was to complement our preliminary in vivo results [7] by a further study using 149Tb for in vitro cell viability assays and in vivo using a well-established tumor mouse model. We employed the recently developed DOTA-folate conjugate (herein referred to as cm09, Figure 1B) and human KB cancer cells which express the folate receptor (FR) at high levels. From our previous in vivo studies performed with 177Lu-cm09, it is known that the novel folate radioconjugate is applicable for preclinical therapy studies in the KB tumor mouse model where it provides an excellent tumor accumulation of radioactivity and as a consequence high antitumor efficacy [15].
2. Experimental
2.1. Chemicals and Reagents for Production and Purification of 149Tb
Gold foils (thickness: 0.1 mm, purity: 99.95%, Goodfellow Cambridge Ltd. Huntingdon, UK) were electrolytically coated with a thin layer of zinc (purity ≥99.9%). The acids in this work (HNO3 suprapur and HCl suprapur) were obtained from VWR International GmbH (Dietikon, Switzerland) and diluted with MilliQ water (18.2 MΩ·cm; Millipore AG, Zug, Switzerland). A solution of NH3 (25%, suprapur) was obtained from VWR International GmbH. α-Hydroxyisobutyric acid (α-HIBA, purity: 99%), L-lactic acid (C3H6O3, purity ≥99%) and NaOH monohydrate (Traceselect, purity ≥99.9995%) were obtained from Sigma-Aldrich International GmbH (St. Gallen, Switzerland).
2.2. Production of 149Tb
149Tb was produced at the isotope separation online facility ISOLDE (CERN, Geneva, Switzerland) as previously reported [7,16,17]. In brief, a tantalum target (50 g/cm2) was irradiated with high-energy (~1.4 GeV) protons. After effusion of the spallation products from the heated target (~2,000 °C) they were ionized by surface ionization and resonant laser ionization. The monocations were extracted from the ion source, accelerated to 50 keV and separated in a magnetic field according to their mass [16,17]. Products of mass number 149 were implanted into a zinc-coated gold foil (70 mm2) and shipped to PSI for the purification procedure.
2.3. Purification of 149Tb
The zinc layer was dissolved in a solution mixture of HNO3/NH4NO3 (0.2 M NO3−, pH 1, 500 μL) at 50 °C. Isolation of 149Tb from isobar and pseudo-isobar impurities and stable Zn was accomplished by cation exchange chromatography. For this purpose a column (5 mm × 35 mm) filled with a strongly acidic macroporous cation exchange resin was employed as recently reported [7,18]. 149Tb and 149Gd were eluted with α-HIBA (0.13 M) at a flow rate of 0.33 mL/min allowing their separation [7]. Further radioactive impurities and stable Zn remained on the column. Regeneration of the column was carried out using higher concentrated α-HIBA (1.0 M). The collected fractions (330 μL each) which contained 149Tb-α-HIBA were acidified by addition of HCl (4 M, 17 μL). The acidic solution was loaded on a second cation exchange chromatography column (4 mm × 4 mm). α-HIBA, NH4+ and HCl were removed by a washing step using MilliQ water. The elution of 149Tb was performed by a solution of L-lactate (0.4 M) previously adjusted to a pH value of 4.7 with sodium hydroxide. The eluted 149Tb-fraction was diluted with the 1.7-fold volume of MilliQ water to adjust the osmolarity to a physiological value (~300 mOsm).
2.4. Preparation of 149Tb-cm09 and Stability in Blood Plasma
Radiolabeling was performed by addition of 18 μL of the DOTA-folate (cm09) stock solution (10 mM, corresp. to 18 nmol of cm09) to the obtained solution of 149Tb (25 MBq) in L-lactate (pH 4.7). The reaction mixture was incubated for 10 min at 95 °C. Quality control was performed by HPLC using a C-18 reversed phase column (Xterra MS C-18, 5 μm, 15 cm × 4.6 cm, Waters, Milford, MA, USA). The mobile phase consisted of MilliQ water with 0.1% trifluoroacetic acid (A) and methanol (B) with a linear gradient from 5% to 80% B over 25 min at a flow rate of 1 mL/min. The product (Rt = 19.5 min) was obtained with a radiochemical purity of >96%. After addition of Na-DTPA (10 μL, 5 mM, pH 5) the labeling solution (1.0–1.2 MBq/nmol) was directly used for in vitro and in vivo application. The stability of 149Tb-cm09 was investigated by incubation of the radioconjugate (50 μL, ~1 MBq) in human plasma (250 μL) at 37 °C. Aliquots (50 μL) were taken for analysis after 30 min, 1 h and 4 h. The proteins were precipitated by addition of methanol (200 μL) and the supernatants were analyzed using HPLC.
2.5. Cell Culture
KB cells (human cervical carcinoma cell line, HeLa subclone; ACC-136) were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Cells were cultured as monolayers at 37 °C in a humidified atmosphere containing 5% CO2. Importantly KB cells were cultured in a folate-free special cell culture medium, FFRPMI (modified RPMI, without folic acid, vitamin B12 and phenol red; Cell Culture Technologies GmbH, Gravesano/Lugano, Switzerland). FFRPMI media was supplemented with 10% heat-inactivated fetal calf serum (FCS), L-glutamine and antibiotics (penicillin/streptomycin/fungizone). Routine culture treatment was performed twice a week.
2.6. In Vitro Cell Viability Studies
Inhibition of KB cell viability was investigated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [19]. The cells were harvested and seeded in 96-well plates at 2.5 × 103 cells per well in a final volume of 200 μL FFRPMI medium with supplements. After 24 h incubation for cell adhesion, the medium was removed and the cells were incubated with 149Tb-cm09 (0.05–500 kBq in 200 μL medium/well) alone or in combination with excess folic acid (200 nM) for 4 h at 37 °C. Cell incubation with FFRPMI medium only was performed as a control experiment. After incubation, the supernatants were removed and KB cells were washed with PBS (200 μL/well) before addition of supplemented FFRPMI medium (200 μL/well). Cells were then allowed to grow for 4 days before analysis as previously reported [20]. After addition of 30 μL of an MTT solution (5 mg/mL) to each well, the well-plates were incubated for an additional 4 h at 37 °C. The medium was removed and the dark-violet formazan crystals were dissolved in dimethyl sulfoxide. The absorbance was determined at 560 nm using a microplate reader (Victor X3, Perkin Elmer, Waltham, MA, USA). To quantify cell viability, the ratio of the absorbance of the test samples to the absorbance of control cell samples (=100% viability) was calculated using Microsoft Excel software (Microsoft Corp. Redmond, WA, USA).
2.7. In Vivo Therapy Studies
In vivo experiments were approved by the local veterinary department and conducted in accordance with the Swiss law of animal protection. Four to five-week-old female, athymic nude mice (CD-1 Foxn-1/nu) were purchased from Charles River Laboratories (Sulzfeld, Germany). They were fed with a folate-deficient rodent diet (ssniff Spezialdiäten GmbH, Soest, Germany) starting 5 days prior to tumor cell inoculation [21]. Endpoint criteria were defined as weight loss of >15% of the initial body weight (at day 0), tumor volume >1,000 mm3, ulceration or bleeding of the tumor xenograft or abnormal behavior indicating pain or unease of the animal.
The therapy study was performed according to the injection protocol shown in Table 1. Twelve mice were subcutaneously inoculated with 5 × 106 KB tumor cells (suspended in 100 μL PBS) as previously reported 4 days before injection of the radioactive folate conjugate [15].

Table 1.
Injection protocol of the in vivo therapy study.
| Number of mice [n] | Injection solution | Injected radioactivity [MBq] | |
|---|---|---|---|
| Group A | 4 | L-lactate solution | - |
| Group B | 4 | 149Tb-cm09 in L-lactate solution | 2.2 |
| Group C | 4 | 149Tb-cm09 in L-lactate solution | 3.0 |
At the time of treatment the tumors reached a volume 60–80 mm3. Mice of group A were intravenously injected with only L-lactate solution whereas mice of group B and C received 149Tb-cm09 (2.2 MBq and 3.0 MBq, respectively) in the same L-lactate solution. The amount of injected activity was the highest possible based on the availability of the nuclide sufficient to inject 4 mice. After start of the therapy mice were weighed three times a week over 35 days and tumor volumes were monitored by measuring two perpendicular diameters with a caliper and calculated according to the equation: V = 0.5 × L × W2 (L is the length (large diameter) and W is the width (small diameter) of the tumor). Mice were removed from the study if one or several of the predefined endpoint criteria were reached which required euthanasia.
The therapeutic efficacy was expressed as the percentage of tumor growth inhibition (%TGI) calculated according to the formula: %TGI = 100 − (RTVT/RTVU × 100), where RTVT is the mean relative tumor volume of treated mice (groups B or C), and RTVU is the mean relative tumor volume of untreated mice (group A) determined at day 21 when the first control mouse was euthanized. The tumor growth delay index (TGDI) was calculated according to the formula: TGDI = TGDT/TGDU. It was defined as the mean tumor growth delay ratio of treated (TGDT) and untreated animals (TGDU) which was required to increase the RTV 5-fold [22].
2.8. Determination of Plasma Parameters
Plasma parameters such as blood urea nitrogen (BUN), alkaline phosphatase (ALP), and total bilirubin (TBIL) were measured from all mice at day 14 after start of the therapy and before euthanasia of each mouse. BUN is a common parameter to determine potential damage to the kidneys whereas increased ALP and TBIL could be an indication for impaired liver function. Plasma samples were prepared by centrifugation of blood samples (150–200 μL per mouse) drawn from the sublingual vein of each mouse and collected in heparinized vials (Microvette, 200 mL Sarstedt, Nümbrecht, Germany). For each parameter a plasma volume of 10 μL was required for the analysis using a Fuji Dri-Chem 40000i analyzer (Polymed Medical Center AG, Glattbrugg, Switzerland).
2.9. Dosimetric Calculations
To estimate the equivalent absorbed dose for 149Tb-cm09 in KB tumor xenografts, biodistribution data obtained with 161Tb-cm09 were used. Based on these data the following calculations were made: (i) the cumulative radioactivity was calculated from integrated AUCs (MBq·s) of biodistribution data expressed in non decay-corrected percent injected activity [%IA] per tumor mass (assumed as 100 mg, due to an approximate calculated volume of the tumors at the start of the therapy); (ii) the adsorbed radiation dose in tumor xenografts was assessed for a sphere of 100 mg using the Unit Density Sphere Model from RADAR [23]; (iii) The absorbed dose (mGy/MBq) was calculated by multiplying the AUC (s; normalized to 1 MBq ID) with the S-value (mGy/MBq·s); and (iv) the dose (mGy) was calculated by multiplying the absorbed dose (mGy/MBq) with the amount of injected radioactivity.
2.10. Statistical Significance
Significance of the survival time and tumor growth delay was calculated by the performance of the t-test (Microsoft Excel). All analyses were two-tailed and considered as type 3 (two sample unequal variance). A p-value < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Production and Purification of 149Tb
149Tb was produced by proton-induced spallation of tantalum targets at ISOLDE/CERN on a zinc-covered gold foil containing isobar and pseudo-isobar nuclides of mass number 149. Upon arrival at PSI (~4 h after the end of collection) ~40 MBq 149Tb, ~5 MBq 149Gd, ~0.4 MBq 145Eu (from α-decay of 149Tb), ~70 MBq 133mCe, ~15 MBq 133Ce and ~350 MBq 133La were detected. The mass 133 pseudo-isobars appear at mass 149 as monoxide molecular ions. The radioactive solution which was obtained after dissolution of the zinc-layer was loaded on column I for chromatographic separation of 149Tb. The elution was accomplished with a diluted solution of α-HIBA within only 25 min. The 149Tb (~29 MBq, corresponding to 73% of total radioactivity) was isolated in a volume of 1.65 mL free of radioactive impurities. Under these isocratic elution conditions an excellent separation of 149Tb and 149Gd was achieved (Figure 2). Further radiolanthanide impurities such as 145Eu, 133mCe, 133Ce and 133La as well as stable Zn were retained on the chromatographic column I and eluted afterwards using a higher concentrated α-HIBA solution for regeneration of the column.
After concentration of the 149Tb-solution by adsorption at the cation exchanger of the second column, the product was obtained within 25 min as 149Tb-L-lactate (0.4 M, pH 4.7) in a small volume of only 225 μL. Addition of 375 μL MilliQ water resulted in a solution of 0.15 M sodium L-lactate with a physiological osmolarity of ~300 mOsm. Determination of radioactivity revealed a total yield of ~25 MBq 149Tb containing ~88 kBq 149Gd—which decays via electron capture and γ-ray emission—as the only detectable radionuclide impurity generated by the decay of 149Tb after separation on column I. The total yield of the separation process was ~63% (corresponding to a ~94% decay-corrected yield) and the whole isolation process including measurement of the single elution fractions was accomplished within 145 min. The L-lactate-based physiological formulation was suitable not only for the direct performance of radiolabeling of cm09 but allowed even in vitro and in vivo application without further purification steps.
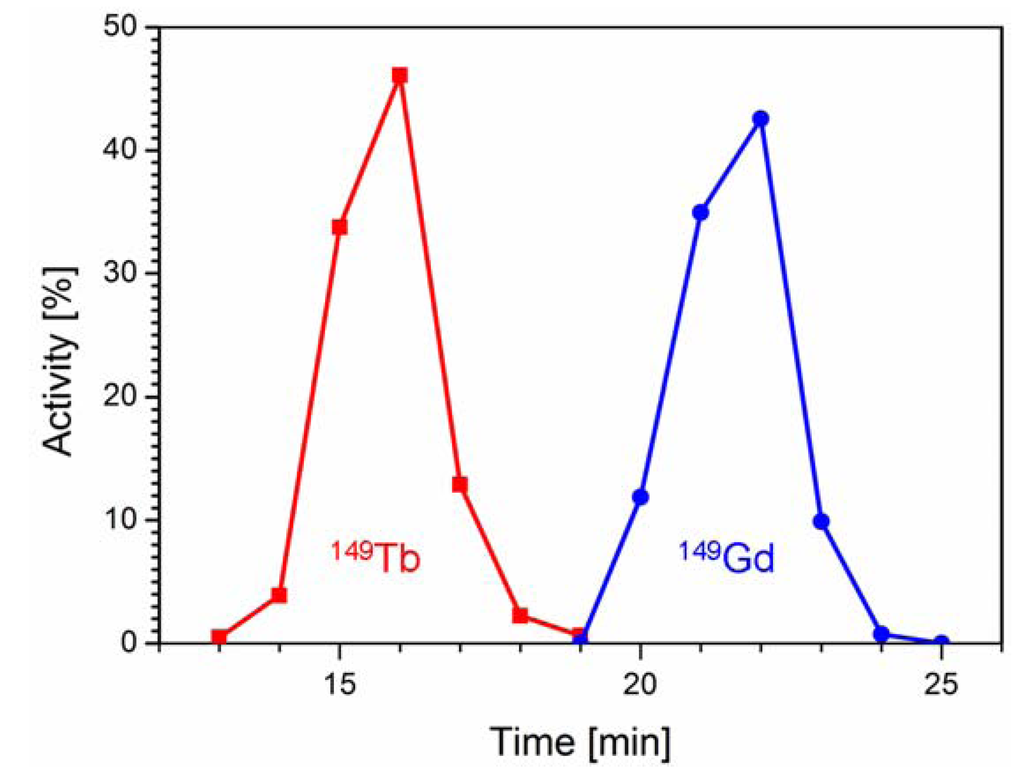
Figure 2.
Elution profile of chromatographic column I showing separation of 149Tb (■, red) from 149Gd (●, blue).
In comparison to our previous study [7] where we used 149Tb for radiolabeling directly after elution from the first column in the α-HIBA solution (~0.15 M), the purification process was optimized in the present study. The advantages were two-fold: Firstly, using the second chromatographic column allowed concentration of the radioactivity in a smaller volume which was favorable for subsequent radiolabeling steps and in vivo application. Secondly, the elution was carried out with a solution of L-lactate providing 149Tb in a more physiological solution compared to the α-HIBA solution which was employed in our previous studies.
3.2. Radiosynthesis of 149Tb-cm09 and Stability in Blood Plasma
Radiolabeling of cm09 (18 nmol) with 149Tb (25 MBq) resulted in a product peak of the radiolabeled product with a retention time (Rt) of 19.5 min. The chromatogram was equal to what was previously obtained with the 161Tb-labeled match (161Tb-cm09) [9]. On the other hand injection of free 149/161Tb(III) was eluted almost with the front (Rt = 3 min). These findings confirmed the identity of the product peak as 149Tb-cm09 which was obtained with a radiochemical purity of >96% with only insignificant traces of free 149Tb(III).
Incubation of 149Tb-cm09 (1.0–1.2 MBq/nmol) in blood plasma at 37 °C revealed high stability (>98%) of the folate radioconjguate over at least 4 hours. These findings were expected as it was previously shown that 161Tb-cm09 was completely stable in human blood plasma over several days [7,9].
3.3. In Vitro Application of 149Tb-cm09
In vitro cell viability assays were performed to investigate the effect of 149Tb-cm09. FR-positive KB tumor cells were incubated with increasing radioactivity concentrations of 149Tb-cm09. It was found that the viability of the KB cells was inhibited in an activity-dependent manner (Figure 3). Application of a low radioactivity concentration of 149Tb-cm09 (0.5 kBq/mL) resulted in an only 20%-reduction of the tumor cell viability. However, a 1,000-fold higher radioactivity concentration (500 kBq/mL) resulted in an almost complete loss of KB cell viability. Addition of excess folic acid to block cell surface exposed FRs abolished the effect of 149Tb-cm09 completely even at high radioactivity concentrations. Under these conditions tumor cells grew normally and comparably to untreated control cells (Figure 3). These findings clearly indicated a FR-specific effect of 149Tb-cm09.
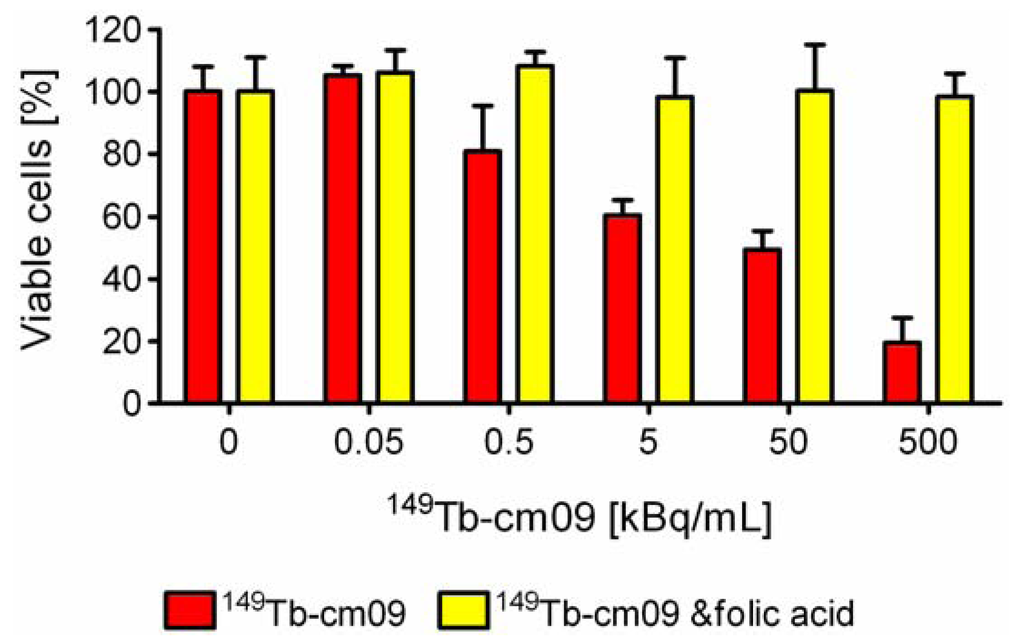
Figure 3.
In vitro viability of KB tumor cells is reduced upon exposure to increasing radioactivity concentrations of 149Tb-cm09 (red bars). Incubation of cells with excess folic acid to block FRs protected KB cells from the 149Tb-cm09-induced inhibitory effect (yellow bars). Exposure of cells to unlabeled cm09 (100 nM to 10 μM) had no effect on cell growth (data not shown).
3.4. Therapy Study in Tumor-Bearing Mice Using 149Tb-cm09
The therapy study in KB tumor-bearing nude mice showed significant tumor growth delay (TGD) in 149Tb-cm09 treated mice compared to untreated control mice (Figure 4A). The tumor growth delay index for a 5-fold increased tumor volume compared to the tumor volume at the beginning of the study (TGDI5) reached a value of 1.5 (p = 0.04) for mice of group B which were treated with 2.2 MBq 149Tb-cm09. This means a 1.5-fold higher value than for untreated control mice (TGDI5 = 1). In mice of group C, which received 3.0 MBq of 149Tb-cm09, the TGDI5 was even 2.0-fold (p = 0.01) increased compared to the TGDI5 of untreated controls. At day 21 when the first control mouse had to be euthanized due to an oversized tumor the average relative tumor volume (RTV) was significantly smaller in treated mice of group B (p = 0.01) and group C (p = 0.001) compared to the tumor volume of untreated control mice (group A). At this time point of the study tumor growth was inhibited by 62% (group B) and 85% (group C), respectively, indicating a dose-dependent therapeutic efficacy of 149Tb-cm09. The average survival time was increased by 45% (30.5 d, p = 0.01) in mice of group B and by 105% (43 d, p = 0.004) in mice of group C compared to control mice (group A, 21 d) (Figure 4B).
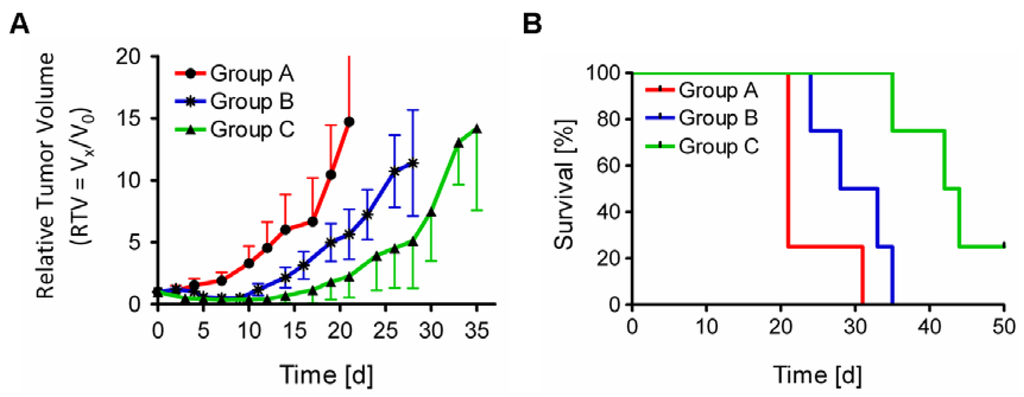
Figure 4.
Results of FR-targeted radionuclide α-therapy using 149Tb-cm09 in KB tumor-bearing mice. (A) Graph of the average relative tumor volume (RTV) of mice from each group (n = 4) during the time period when at least 3 mice were still alive; (B) Survival curves of mice from each group (group A: 21 d; group B: 30.5 d and group C: 43 d).
Analysis of blood plasma parameters (BUN, ALP and TBIL) did not reveal significant changes (p > 0.05) among control mice and mice treated with 149Tb-cm09 (Table 2). This analysis indicated unimpaired renal and hepatobiliar function. Hence, it can be concluded that acute radiotoxic effects to the kidneys and the liver were not experienced by FR-targeted α-radionuclide therapy over the whole time of this study which lasted for 35 days. More detailed studies to investigate effects of 149Tb-cm09 on kidney function will be necessary since undesired side-effects on the long-term cannot be excluded based on the examinations performed herein.

Table 2.
Results of plasma analysis at day 14 and before terminal (in parentheses); (BUN = blood urea nitrogen, ALP = alkaline phosphatase, TBIL = total bilirubin).
| BUN [mmol/L] | ALP [U/L] | TBIL [μmol/L) | |
|---|---|---|---|
| Group A | 7.0 ± 1.8 (4.1 ± 0.5) | 77 ± 10 (99 ± 16) | 6.5 ± 2.5 (6.3 ± 1.0) |
| Group B | 4.6 ± 0.6 (4.7 ± 0.4) | 67 ± 5 (119 ± 7) | 7.6 ± 4.7 (6.0 ± 1.0) |
| Group C | 5.2 ± 1.3 (5.9 ± 1.4) | 75 ± 14 (73 ± 21) | 7.0 ± 0.8 (5.0 ± 1.0) |
Overall the present results outperformed our previous data which were obtained upon administration of two injections of 149Tb-cm09 at low quantities of radioactivity (1.1 MBq and 1.3 MBq, respectively) [7]. Compared to our previous therapy study, the present study design differed in that the mice received the whole amount of radioactivity in a single injection of either 2.2 MBq 149Tb-cm09 (group B) or 3.0 MBq 149Tb-cm09 (group C).
Beyer et al. conducted an experiment where 149Tb-labeled rituximab (5.5 MBq per mouse) was investigated in a leukemia animal model using SCID mice with an intravenous graft of Daudi cells [5]. Their aim was to examine the efficacy of 149Tb-rituximab to specifically kill circulating single cancer cells or small cell clusters in vivo. The therapy was started within 3 days upon intravenous xenografting of a lethal number of Daudi cells when most of the tumor cells were expected to be still in circulation [5]. Whereas untreated control mice had to be euthanized within the first 37 days a tumor-free survival was found for over 120 d in almost 90% of the 149Tb-rituximab treated animals. Since application of the same amount of unlabeled rituximab did not show a therapeutic effect, the favorable outcome of this study could be ascribed to the α-radionuclide therapy [5]. In the present study injection of 149Tb-cm09 reduced tumor growth of solid KB xenografts and increased the survival time significantly in mice of both treated groups (B and C) compared to untreated control mice (group A). These excellent results complement those of Beyer et al. by demonstrating the therapeutic potential of 149Tb. However, our study design differed from that of Beyer et al. in three respects. (i) Instead of using an established antibody, we employed a small-molecular weight folate conjugate (cm09) as a targeting agent which previously proved the promising potential to be used for therapeutic purposes [15]; (ii) The amount of injected radioactivity was 2.2 MBq and 3.0 MBq, respectively, in our study compared to 5.5 MBq which were used in the study of Beyer et al. [5]; (iii) Finally the tumor mouse model which was used in our study was based on solid tumor xenografts of a human cervical cancer cell line while Beyer et al. used a tumor mouse model with circulating leukemia cells.
3.5. Dosimetric Calculations
In order to obtain an idea about the radioactive dose burden of 149Tb-cm09 to KB tumor xenografts a dose estimation was made while taking only the self-radiation dose into account. According to the AUC obtained from tissue distribution data in mice injected with 161Tb-cm09 [7] and the S-value of 149Tb listed for a sphere of 100 mg an absorbed dose of 8.67 Gy/MBq was estimated for KB tumor xenografts. This resulted in an absorbed dose of ~19 Gy (group B) and ~26 Gy (group C) in tumors upon a single injection of 2.2 MBq and 3.0 MBq of 149Tb-cm09, respectively.
Recently, we peformed a preclinical therapy study with 10 MBq of 161Tb-cm09 using the same KB tumor mouse model [9]. In that case the dose to the tumor was calculated to a value of ~33 Gy and the corresponding TGDI5 reached a value of ~2.2. In the present study the TGDI5 for 149Tb-cm09 was ~1.6 (2.2 MBq, ~19 Gy) and ~2.0 (3.0 MBq, ~26 Gy) indicating a slightly improved effect of the α-radionuclide therapy. However, for an exact comparison of α- and β−/Auger-radionuclide therapy it would be necessary to perform a site-by-site study using 149Tb-folate and 161Tb-folate with an activity that results in the same calculated dose to the tumor.
4. Conclusions
In this study the potential of α-radionuclide therapy in general and of 149Tb in particular was demonstrated using a folate-based biomolecule. Application of 149Tb-cm09 was well-tolerated in mice and treated KB tumor xenografts efficiently. The experiments revealed significant tumor growth delay and an increased survival time of mice treated with 149Tb-cm09 compared to untreated control mice. However, further studies will be required to investigate potential advantages of FR-targeted α-radionuclide therapy over β−-radionuclide therapy with regard to tumor response and potential damage to the kidneys.
Acknowledgments
We thank Nadja Romano, Maruta Bunka, the ISOLDE technical team and the ISOLDE RILIS team for technical assistance. This project was financially supported by the Swiss National Science Foundation (Ambizione Grant), COST (Action BM0607), the Swiss Cancer League (KLS-02762-02-2011), the Swiss South African Joint Research Programme (SSAJRP) and by the European Union via the ENSAR Project (contract 262010).
Author Contributions
C.M. coordinated the study, supervised the experiments and wrote the manuscript. J.R. and S.H. performed the in vitro and in vivo studies at PSI. H.D. performed the separation of 149Tb at PSI. U.K. and K.J. coordinated and performed the production of 149Tb at ISOLDE/CERN. K.Z. developed the separation procedure of 149Tb and contributed to the study design. A.T. and R.S. gave advice in the interpretation of the data and critically reviewed the manuscript. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oyen, W.J.; Bodei, L.; Giammarile, F.; Maecke, H.R.; Tennvall, J.; Luster, M.; Brans, B. Targeted therapy in nuclear medicine-current status and future prospects. Ann. Oncol. 2007, 18, 1782–1792. [Google Scholar] [CrossRef]
- Zoller, F.; Eisenhut, M.; Haberkorn, U.; Mier, W. Endoradiotherapy in cancer treatment—Basic concepts and future trends. Eur. J. Pharmacol. 2009, 625, 55–62. [Google Scholar] [CrossRef]
- Allen, B.J.; Blagojevic, N. Alpha- and beta-emitting radiolanthanides in targeted cancer therapy: The potential role of terbium-149. Nucl. Med. Commun. 1996, 17, 40–47. [Google Scholar] [CrossRef]
- Allen, B.J. Targeted alpha therapy: Evidence for potential efficacy of alpha-immunoconjugates in the management of micrometastatic cancer. Australas. Radiol. 1999, 43, 480–486. [Google Scholar]
- Beyer, G.J.; Miederer, M.; Vranjes-Duric, S.; Comor, J.J.; Kunzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef]
- Beyer, G.J.; Comor, J.J.; Dakovic, M.; Soloviev, D.; Tamburella, C.; Hagebo, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Starodub, G.Y.; et al. Production routes of the alpha emitting 149Tb for medical application. Radiochim. Acta. 2002, 90, 247–252. [Google Scholar]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; van der Walt, N.T.; Türler, A.; Schibli, R. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α- and β−-radionuclide therapy: An in vivo proof-of-concept study with a new receptor-targeted folate derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef]
- Lehenberger, S.; Barkhausen, C.; Cohrs, S.; Fischer, E.; Grünberg, J.; Hohn, A.; Koster, U.; Schibli, R.; Türler, A.; Zhernosekov, K. The low-energy beta- and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl. Med. Biol. 2011, 38, 917–924. [Google Scholar]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Bernhardt, P.; Zhernosekov, K.; Türler, A.; Schibli, R. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 476–485. [Google Scholar] [CrossRef]
- Imam, S.K. Advancements in cancer therapy with alpha-emitters: A review. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 271–278. [Google Scholar] [CrossRef]
- Miederer, M.; Seidl, C.; Beyer, G.J.; Charlton, D.E.; Vranjes-Duric, S.; Comor, J.J.; Huber, R.; Nikula, T.; Apostolidis, C.; Schuhmacher, C.; et al. Comparison of the radiotoxicity of two alpha-particle-emitting immunoconjugates, terbium-149 and bismuth-213, directed against a tumor-specific, exon 9 deleted (d9) e-cadherin adhesion protein. Radiat. Res. 2003, 159, 612–620. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The chemical fate of 212Bi-DOTA formed by β− decay of 212Pb-DOTA2−. Radiochim. Acta 1993, 60, 1–10. [Google Scholar]
- Schwartz, J.; Jaggi, J.S.; O’Donoghue, J.A.; Ruan, S.; McDevitt, M.; Larson, S.M.; Scheinberg, D.A.; Humm, J.L. Renal uptake of bismuth-213 and its contribution to kidney radiation dose following administration of actinium-225-labeled antibody. Phys. Med. Biol. 2011, 56, 721–733. [Google Scholar]
- Wilbur, D.S. Enigmatic astatine. Nat. Chem. 2013, 5, 246. [Google Scholar] [CrossRef]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Allen, B.J.; Goozee, G.; Sarkar, S.; Beyer, G.; Morel, C.; Byrne, A.P. Production of terbium-152 by heavy ion reactions and proton induced spallation. Appl. Radiat. Isot. 2001, 54, 53–58. [Google Scholar] [CrossRef]
- Köster, U.; Collaboration ISOLDE. Isolde target and ion source chemistry. Radiochim. Acta 2001, 89, 749–756. [Google Scholar]
- Müller, C.; Fischer, E.; Behe, M.; Köster, U.; Dorrer, H.; Reber, J.; Haller, S.; Cohrs, S.; Blanc, A.; Grünberg, J.; et al. Future prospects for SPECT imaging using the radiolanthanide terbium-155—Production and preclinical evaluation in tumor-bearing mice. Nucl. Med. Biol. 2013. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reber, J.; Struthers, H.; Betzel, T.; Hohn, A.; Schibli, R.; Müller, C. Radioiodinated folic acid conjugates: Evaluation of a valuable concept to improve tumor-to-background contrast. Mol. Pharm. 2012, 9, 1213–1221. [Google Scholar]
- Mathias, C.J.; Wang, S.; Lee, R.J.; Waters, D.J.; Low, P.S.; Green, M.A. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J. Nucl. Med. 1996, 37, 1003–1008. [Google Scholar]
- Sanceau, J.; Poupon, M.F.; Delattre, O.; Sastre-Garau, X.; Wietzerbin, J. Strong inhibition of ewing tumor xenograft growth by combination of human interferon-alpha or interferon-beta with ifosfamide. Oncogene 2002, 21, 7700–7709. [Google Scholar] [CrossRef]
- Radiation Dose Assessment Resource (RADAR). Available online: www.doseinfo-radar.com (accessed on 7 March 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).