Phospho-Chitooligosaccharides below 1 kDa Inhibit HIV-1 Entry In Vitro
Abstract
:1. Introduction
2. Materials and Methods
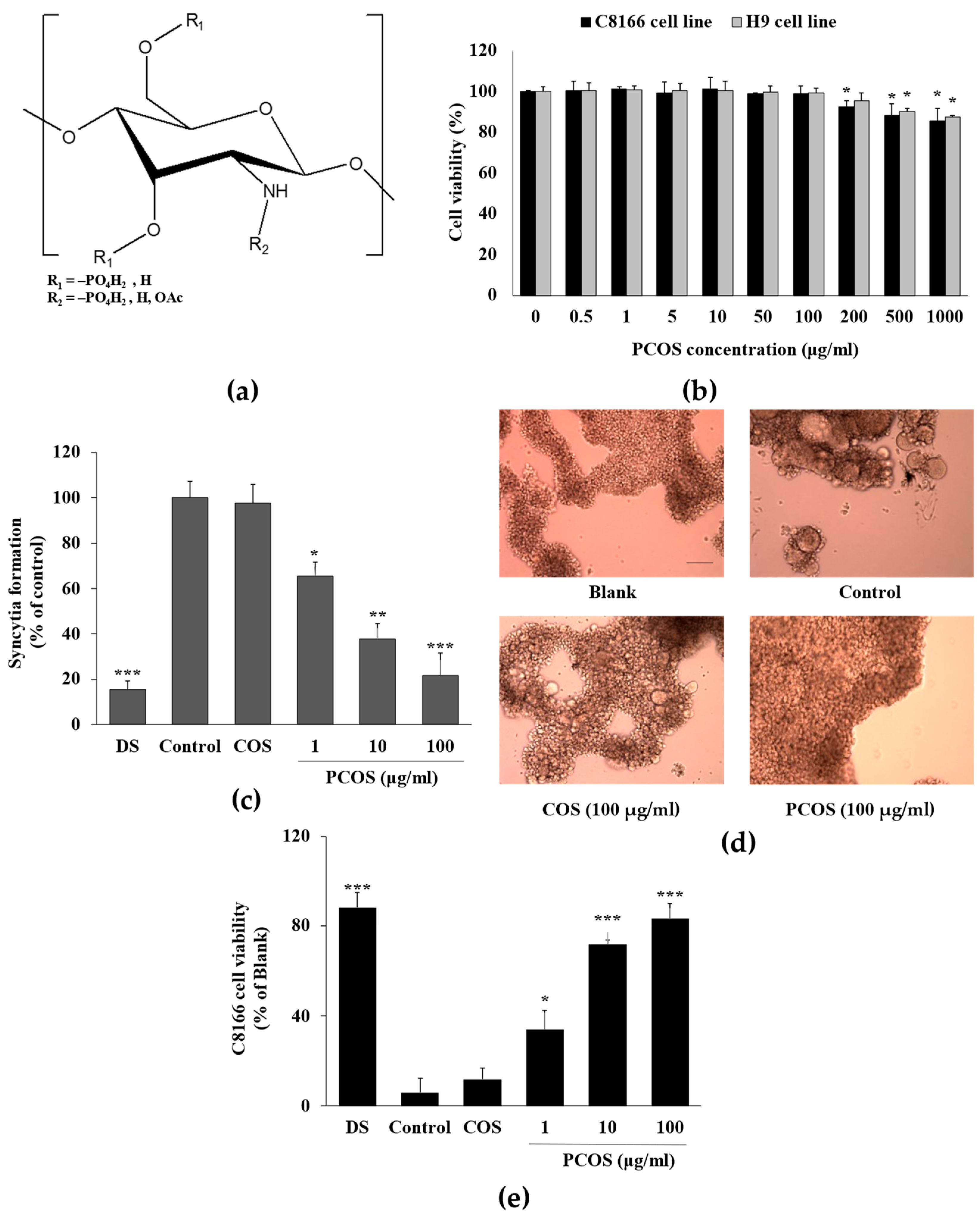
2.1. Materials and PCOS Synthesis
2.2. Cell Culture, HIV-1 Infection, Syncytia Formation, and Cell Viability Analysis
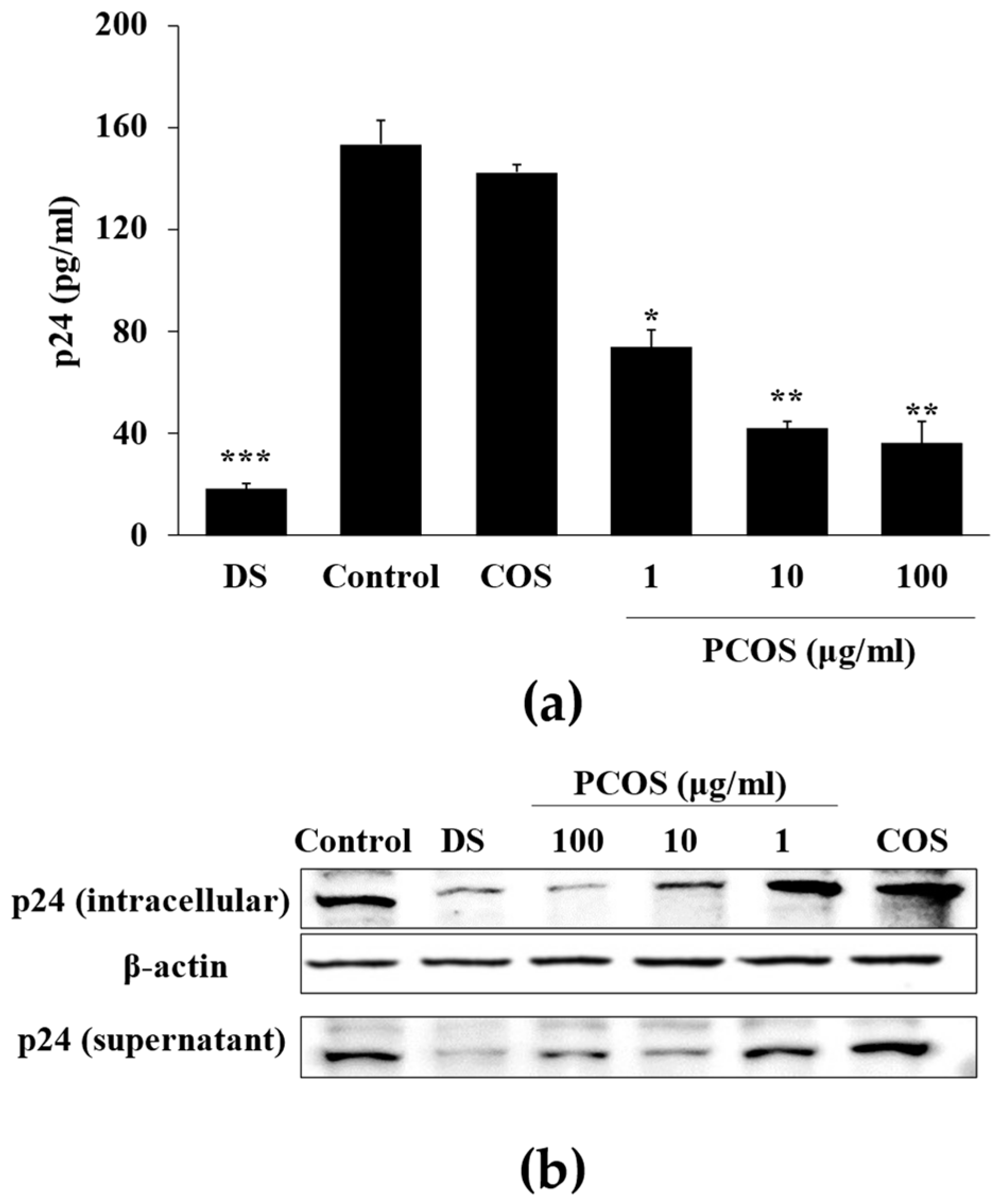
2.3. Measurement of p24 Antigen
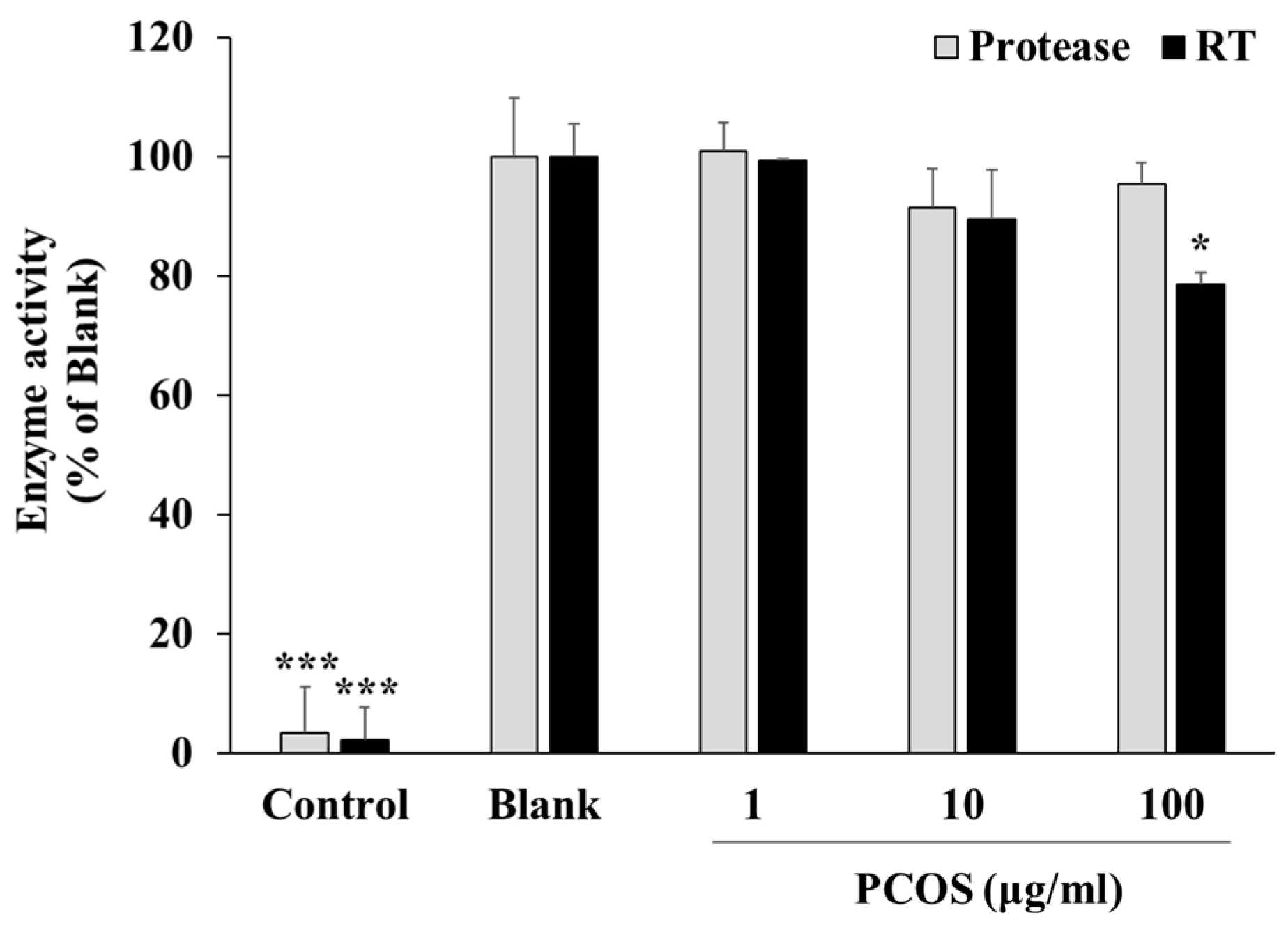
2.4. HIV-1 RT and Protease Activity Assay
2.5. Post-Infection Treatment and Co-Culture Assays
2.6. Analysis of gp120-CD4 Binding
2.7. Statistical Analysis
3. Results and Discussion
3.1. Anti-HIV Activity of PCOS
3.2. Mechanism of Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Sen, I.K.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agric. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A. Microbial valorization of shrimp byproducts via the production of thermostable chitosanase and antioxidant chitooligosaccharides. Biocatal. Agric. Biotechnol. 2019, 20, 101269. [Google Scholar] [CrossRef]
- Zhao, M.; Gu, L.; Li, Y.; Chen, S.; You, J.; Fan, L.; Wang, Y.; Zhao, L. Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr. Polym. 2019, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Lillo, L.; Alarcón, J.; Cabello, G.; Céspedes, C.; Caro, C. Antibacterial Activity of Chitooligosaccharides. Z. Für Naturforschung C 2008, 63, 644–648. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Li, D.; Wang, S.; Zhao, W.; Lv, Z.; Li, X.; Li, H.; Han, Y. Enzymatic preparation of chitooligosaccharides and their anti-obesity application. Biosci. Biotechnol. Biochem. 2020, 84, 1460–1466. [Google Scholar] [CrossRef]
- Vo, T.-S.; Kong, C.-S.; Kim, S.-K. Inhibitory effects of chitooligosaccharides on degranulation and cytokine generation in rat basophilic leukemia RBL-2H3 cells. Carbohydr. Polym. 2011, 84, 649–655. [Google Scholar] [CrossRef]
- Ju, C.; Yue, W.; Yang, Z.; Zhang, Q.; Yang, X.; Liu, Z.; Zhang, F. Antidiabetic Effect and Mechanism of Chitooligosaccharides. Biol. Pharm. Bull. 2010, 33, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, J. Chitooligosaccharides prevent osteopenia by promoting bone formation and suppressing bone resorption in ovariectomised rats: Possible involvement of COX-2. Nat. Prod. Res. 2015, 29, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; De Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-Inflammatory Activity of Chitooligosaccharides in Vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, M.; Ahn, C.-B.; Je, J.-Y. Hepatoprotective effect of chitooligosaccharides against tert-butylhydroperoxide-induced damage in Chang liver cells. Carbohydr. Polym. 2011, 83, 995–1000. [Google Scholar] [CrossRef]
- Despras, G.; Alix, A.; Urban, D.; Vauzeilles, B.; Beau, J.-M. From Chitin to Bioactive Chitooligosaccharides and Conjugates: Access to Lipochitooligosaccharides and the TMG-chitotriomycin. Angew. Chem. 2014, 126, 12106–12110. [Google Scholar] [CrossRef]
- Eom, T.-K.; Senevirathne, M.; Kim, S.-K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Kim, S.-K. Effect of Antimicrobial Activity by Chitosan Oligosaccharide N-Conjugated with Asparagine. J. Microbiol. Biotechnol. 2001, 11, 281–286. [Google Scholar]
- Rajapakse, N.; Kim, M.-M.; Mendis, E.; Huang, R.; Kim, S.-K. Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9 expression in human fibrosarcoma cells via down-regulation of AP-1. Biochim. Biophys. Acta 2006, 1760, 1780–1788. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Y.; Huang, L.; Wang, S.; Hao, C.; Sun, L.; Zhang, Y.; Wang, W.; Li, C. The inhibition effects and mechanisms of sulfated chitooligosaccharides on influenza A virus in vitro and in vivo. Carbohydr. Polym. 2022, 286, 119316. [Google Scholar] [CrossRef]
- Kim, S.; Park, P.; Jung, W.; Byun, H.; Mendis, E.; Cho, Y. Inhibitory activity of phosphorylated chitooligosaccharides on the formation of calcium phosphate. Carbohydr. Polym. 2005, 60, 483–487. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Desroches Le Foll, M. Advances in chitooligosaccharides chemical modifications. Biopolymers 2021, 112, e23461. [Google Scholar] [CrossRef] [PubMed]
- Alvi, Y.; Khalique, N.; Ahmad, A.; Sameen, S. A Study on Side Effect of Antiretroviral Therapy among People living with HIV/AIDS. J. Pharmacovigil. Drug Saf. 2019, 16, 22–25. [Google Scholar]
- Wu, H.; Morris-Natschke, S.L.; Xu, X.; Yang, M.; Cheng, Y.; Yu, S.; Lee, K. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Wang, S.; Deng, R.; Luo, D.; Luo, M.; Huang, Q.; Yu, S.; Pu, C.; Liu, Y.; et al. Recent Advances in the Discovery and Development of Anti-HIV Natural Products. Am. J. Chin. Med. 2022, 50, 1173–1196. [Google Scholar] [CrossRef] [PubMed]
- Cary, D.C.; Peterlin, B.M. Natural Products and HIV/AIDS. AIDS Res. Hum. Retroviruses 2018, 34, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Serna-Arbeláez, M.S.; Florez-Sampedro, L.; Orozco, L.P.; Ramírez, K.; Galeano, E.; Zapata, W. Natural Products with Inhibitory Activity against Human Immunodeficiency Virus Type 1. Adv. Virol. 2021, 2021, 5552088. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Lv, H.-X.; Zhang, Z.-H.; Wang, X.-P.; Cheng, Q.-Q.; Wang, W.; Huang, X.-H.; Zhou, J.-P.; Zhang, Q.; Hou, L.-L.; Huo, W. A Biomimetic Chitosan Derivates: Preparation, Characterization and Transdermal Enhancement Studies of N-Arginine Chitosan. Molecules 2011, 16, 6778–6790. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Haji Molla Hoseini, M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pangestuti, R.; Qian, Z.-J.; Ryu, B.; Kim, S.-K. Biocompatibility and Alkaline Phosphatase Activity of Phosphorylated Chitooligosaccharides on the Osteosarcoma MG63 Cell Line. J. Funct. Biomater. 2010, 1, 3–13. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nagahama, H.; Furuike, T.; Tamura, H. Synthesis of phosphorylated chitosan by novel method and its characterization. Int. J. Biol. Macromol. 2008, 42, 335–339. [Google Scholar] [CrossRef]
- Neurath, A.R.; Strick, N.; Li, Y.-Y.; Debnath, A.K. Punica granatum (Pomegranate) juice provides an HIV-1 entry inhibitor and candidate topical microbicide. BMC Infect. Dis. 2004, 4, 41. [Google Scholar] [CrossRef]
- Battulga, T.; Tumurbaatar, O.; Ganzorig, O.; Ishimura, T.; Kanamoto, T.; Nakashima, H.; Miyazaki, K.; Yoshida, T. Analysis of interaction between sulfated polysaccharides and HIV oligopeptides by surface plasmon resonance. Int. J. Biol. Macromol. 2019, 125, 909–914. [Google Scholar] [CrossRef]
- Sharma, A.; Shahid, A.; Banerjee, R.; Kumar, K.J. Emerging insights into the structure-activity relationship of water-soluble polysaccharides in antiviral therapy. Eur. J. Med. Chem. Rep. 2024, 10, 100122. [Google Scholar] [CrossRef]
- Lee, D.-S.; Jung, K.-E.; Yoon, C.-H.; Lim, H.; Bae, Y.-S. Newly Designed Six-Membered Azasugar Nucleotide-Containing Phosphorothioate Oligonucleotides as Potent Human Immunodeficiency Virus Type 1 Inhibitors. Antimicrob. Agents Chemother. 2005, 49, 4110–4120. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Yang, S.; Zhao, X.; Yi, X. Antiviral activity of phosphorylated Radix Cyathulae officinalis polysaccharide against Canine Parvovirus in vitro. Int. J. Biol. Macromol. 2017, 99, 511–518. [Google Scholar] [CrossRef]
- Ming, K.; Chen, Y.; Yao, F.; Shi, J.; Yang, J.; Du, H.; Wang, X.; Wang, Y.; Liu, J. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017, 94, 28–35. [Google Scholar] [CrossRef]
- Copeland, R.A.; Harpel, M.R.; Tummino, P.J. Targeting enzyme inhibitors in drug discovery. Expert Opin. Ther. Targets 2007, 11, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T. Anti-HIV Mechanism of Sulfated Poly and Oligosaccharides. J. Fiber Sci. Technol. 2020, 76, 387–402. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Crublet, E.; Andrieu, J.-P.; Vivès, R.R.; Lortat-Jacob, H. The HIV-1 Envelope Glycoprotein gp120 Features Four Heparan Sulfate Binding Domains, Including the Co-receptor Binding Site. J. Biol. Chem. 2008, 283, 15193–15200. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadeniz, F.; Kim, S.-K. Phospho-Chitooligosaccharides below 1 kDa Inhibit HIV-1 Entry In Vitro. Curr. Issues Mol. Biol. 2024, 46, 3729-3740. https://doi.org/10.3390/cimb46040232
Karadeniz F, Kim S-K. Phospho-Chitooligosaccharides below 1 kDa Inhibit HIV-1 Entry In Vitro. Current Issues in Molecular Biology. 2024; 46(4):3729-3740. https://doi.org/10.3390/cimb46040232
Chicago/Turabian StyleKaradeniz, Fatih, and Se-Kwon Kim. 2024. "Phospho-Chitooligosaccharides below 1 kDa Inhibit HIV-1 Entry In Vitro" Current Issues in Molecular Biology 46, no. 4: 3729-3740. https://doi.org/10.3390/cimb46040232




