Adverse Skeletal Muscle Adaptations in Individuals Born Preterm—A Comprehensive Review
Abstract
:1. Introduction
2. Abnormal Insulin Signaling and Low IGF Levels
3. Impaired Amino Acid Signaling and Protein Synthesis
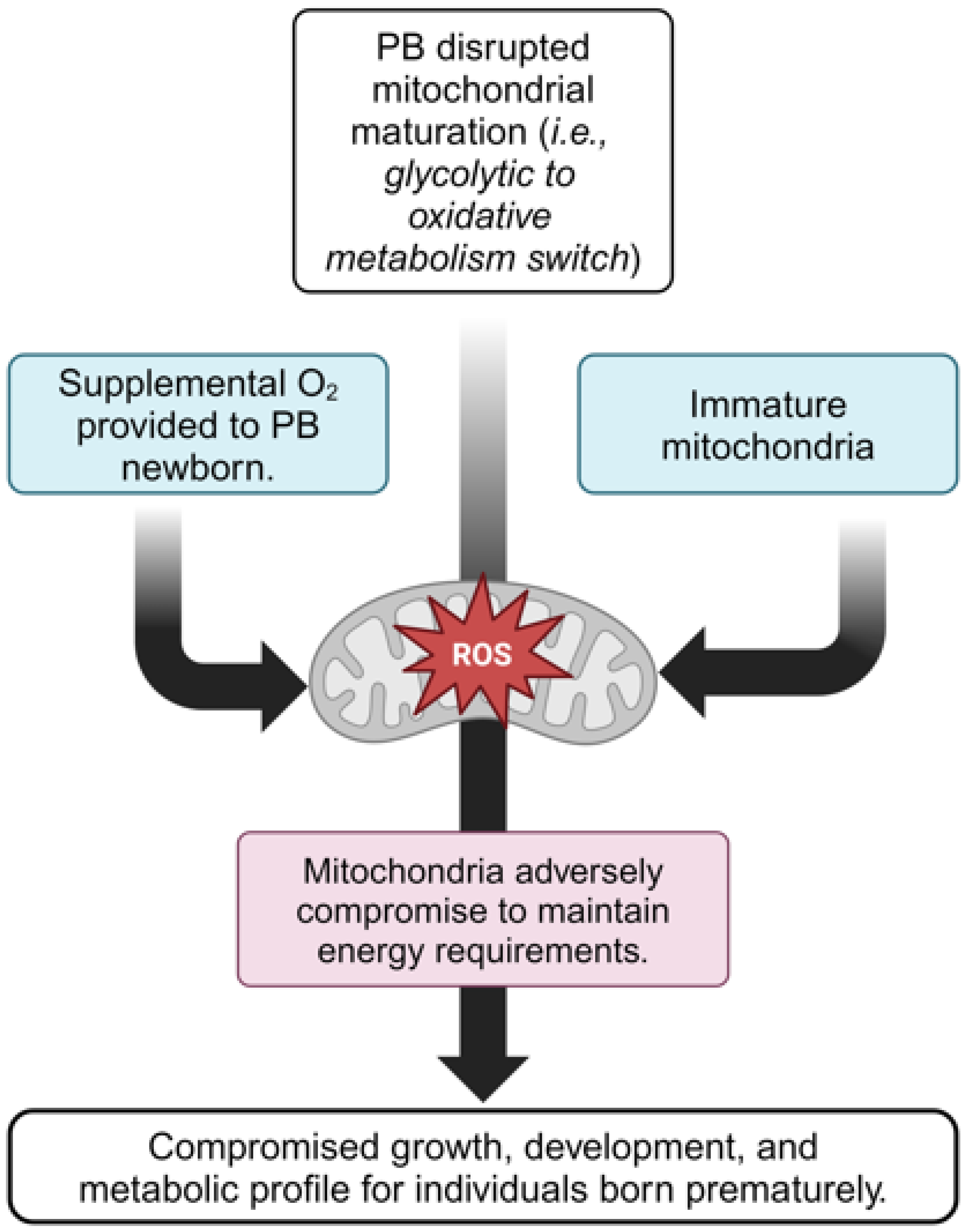
4. Poorly Developed Mitochondria and Associated Inflammation
5. Epigenetic Changes
6. Conclusions
7. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Gough, A.; Spence, D.; Linden, M.; Halliday, H.L.; McGarvey, L.P. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: A systematic review. Chest 2012, 141, 1554–1567. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, H.; Anderson, P.J.; Doyle, L.W.; Woodward, L.J.; Neil, J.J.; Inder, T.E. Brain injury and altered brain growth in preterm infants: Predictors and prognosis. Pediatrics 2014, 134, e444–e453. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. Oxygen toxicity in premature infants. Toxicol. Appl. Pharmacol. 2002, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef]
- Martinez-Zamora, M.D.; Valenzuela, P.L.; Esteban Díez, I.; Martínez-de-Quel, Ó. Influence of preterm birth on physical fitness in early childhood. Eur. J. Sport. Sci. 2023, 23, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Datar, A.; Jacknowitz, A. Birth weight effects on children’s mental, motor, and physical development: Evidence from twins data. Matern. Child Health J. 2009, 13, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Shima, N.; Nakamura, K.; Nitta, O.; Ikai, T. Walking Attainment in Very Low Birth Weight Infants in Japan. Phys. Ther. Res. 2021, 24, 204–210. [Google Scholar] [CrossRef]
- Yaari, M.; Mankuta, D.; Harel-Gadassi, A.; Friedlander, E.; Bar-Oz, B.; Eventov-Friedman, S.; Maniv, N.; Zucker, D.; Yirmiya, N. Early developmental trajectories of preterm infants. Res. Dev. Disabil. 2018, 81, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Sundquist, K. Preterm birth and risk of type 1 and type 2 diabetes: A national cohort study. Diabetologia 2020, 63, 508–518. [Google Scholar] [CrossRef]
- Jensen, C.B.; Storgaard, H.; Madsbad, S.; Richter, E.A.; Vaag, A.A. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J. Clin. Endocrinol. Metab. 2007, 92, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.-K. Preterm birth and risk of heart failure up to early adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.N.; Haraldsdottir, K.; Beshish, A.G.; Barton, G.P.; Watson, A.M.; Palta, M.; Chesler, N.C.; Francois, C.J.; Wieben, O.; Eldridge, M.W. Association between preterm birth and arrested cardiac growth in adolescents and young adults. JAMA Cardiol. 2020, 5, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.M.; Ramsingh, L.; Gunn, E.; Streiner, D.; Van Lieshout, R.; Boyle, M.; Gerstein, H.; Schmidt, L.; Saigal, S. Cardiometabolic Health in Adults Born Premature With Extremely Low Birth Weight. Pediatrics 2016, 138, e20160515. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.; Pätsi, P.; Leskinen, M.; Björkman, K.; Kulmala, P.; Tulppo, M.P.; Valkama, M.; Ojaniemi, M. Preterm-Born Young Women Have Weaker Hand Grip Strength Compared to Their Full-Term-Born Peers. Children 2023, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.S.; Dichtel, L.E.; Santoso, K.; Torriani, M.; Miller, K.K.; Bredella, M.A. Association between muscle mass and insulin sensitivity independent of detrimental adipose depots in young adults with overweight/obesity. Int. J. Obes. 2020, 44, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Naberhuis, J.K.; Suryawan, A.; Nguyen, H.V.; Hernandez-Garcia, A.; Cruz, S.M.; Lau, P.E.; Olutoye, O.O.; Stoll, B.; Burrin, D.G.; Fiorotto, M.L.; et al. Prematurity blunts the feeding-induced stimulation of translation initiation signaling and protein synthesis in muscle of neonatal piglets. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E839–E851. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Rudar, M.; Naberhuis, J.K.; Fiorotto, M.L.; Davis, T.A. Preterm birth alters the feeding-induced activation of Akt signaling in the muscle of neonatal piglets. Pediatr. Res. 2023, 93, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Rehman Mian, M.O.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M. Early pulmonary vascular disease in young adults born preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Huckstep, O.J.; Williamson, W.; Telles, F.; Burchert, H.; Bertagnolli, M.; Herdman, C.; Arnold, L.; Smillie, R.; Mohamed, A.; Boardman, H. Physiological stress elicits impaired left ventricular function in preterm-born adults. J. Am. Coll. Cardiol. 2018, 71, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.T.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Gust, C.E.; Mangum, T.S.; Gladstone, I.M.; Duke, J.W. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann. Am. Thorac. Soc. 2014, 11, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Lamata, P.; Williamson, W.; Alsharqi, M.; Tan, C.M.J.; Burchert, H.; Huckstep, O.J.; Suriano, K.; Francis, J.M.; Pelado, J.L.; et al. Multimodality Imaging Demonstrates Reduced Right-Ventricular Function Independent of Pulmonary Physiology in Moderately Preterm-Born Adults. JACC Cardiovasc. Imaging 2020, 13, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.; Cutfield, W.S.; Derraik, J.G.; Dalziel, S.R.; Harding, J.E.; Robinson, E.; Biggs, J.; Jefferies, C.; Hofman, P.L. Insulin sensitivity and β-cell function in adults born preterm and their children. Diabetes 2012, 61, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.L.; Tinnion, R.; Hollingsworth, K.G.; Trenell, M.I.; Pearce, M.S.; Cheetham, T.D.; Embleton, N.D. Muscle Function, Body Composition, Insulin Sensitivity and Physical Activity in Adolescents Born Preterm: Impact of Gestation and Vitamin D Status. Nutrients 2022, 14, 5045. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Katayama, D.; Hara, K.; Sato, Y.; Tanabe, S.; Aoki, M.; Aoki, R.; Morioka, I. Percentile-Based Reference Values of Umbilical Cord Blood Insulin-like Growth Factor 1 in Japanese Newborns. J. Clin. Med. 2022, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Keens, T.G.; Bryan, A.C.; Levison, H.; Ianuzzo, C.D.; Riddell, M.C.; Taylor, B.J.; How, S.C.; Romer, L.M.; Smith, D.; Green, H.; et al. Developmental pattern of muscle fiber types in human ventilatory muscles. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 909–913. [Google Scholar] [CrossRef]
- Manferdelli, G.; Narang, B.J.; Pialoux, V.; Giardini, G.; Debevec, T.; Millet, G.P. Microvascular and oxidative stress responses to acute high-altitude exposure in prematurely born adults. Sci. Rep. 2023, 13, 6860. [Google Scholar] [CrossRef]
- Fan, L.; Cacicedo, J.M.; Ido, Y. Impaired nicotinamide adenine dinucleotide (NAD+) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment. J. Diabetes Investig. 2020, 11, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Lin, H.-Y.; Wang, C.-H.; Su, B.-H.; Lin, C.-C. Association of preterm birth and small for gestational age with metabolic outcomes in children and adolescents: A population-based cohort study from Taiwan. Pediatr. Neonatol. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Tian, H.; Liu, Z.; Yin, X.; Xi, B. Preterm birth and risk of type 1 and type 2 diabetes: Systematic review and meta-analysis. Obes. Rev. 2014, 15, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Hovi, P.; Andersson, S.; Eriksson, J.G.; Järvenpää, A.-L.; Strang-Karlsson, S.; Mäkitie, O.; Kajantie, E. Glucose regulation in young adults with very low birth weight. N. Engl. J. Med. 2007, 356, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Osmond, C.; Barker, D.J.; Eriksson, J.G. Preterm birth—A risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care 2010, 33, 2623–2625. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Mackenzie, T.A.; Jones, J.D.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 National Health and Nutrition Examination Survey. Clin. Nutr. 2016, 35, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Metter, E.J.; Ramachandran, R.; Chia, C.W.; Saudek, C.D.; Ferrucci, L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.M. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: A cross-sectional study. Sci. Rep. 2018, 8, 2703. [Google Scholar] [CrossRef]
- Maestro, B.; Campión, J.; Dávila, N.; Calle, C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr. J. 2000, 47, 383–391. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Rad, E.; Djalali, M.; Koohdani, F.; Saboor-Yaraghi, A.A.; Eshraghian, M.R.; Javanbakht, M.H.; Saboori, S.; Zarei, M.; Hosseinzadeh-Attar, M.J. The Effects of Vitamin D Supplementation on Glucose Control and Insulin Resistance in Patients with Diabetes Type 2: A Randomized Clinical Trial Study. Iran. J. Public Health 2014, 43, 1651–1656. [Google Scholar] [PubMed]
- Jamka, M.; Woźniewicz, M.; Jeszka, J.; Mardas, M.; Bogdański, P.; Stelmach-Mardas, M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: Systematic review with meta-analysis. Sci. Rep. 2015, 5, 16142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Guo, H.; Meng, K.; Qiu, J.; Benardot, D. Muscle-Related Effect of Whey Protein and Vitamin D3 Supplementation Provided before or after Bedtime in Males Undergoing Resistance Training. Nutrients 2022, 14, 2289. [Google Scholar] [CrossRef] [PubMed]
- Heslin, M.; Newman, E.; Wolf, R.; Pisters, P.; Brennan, M. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am. J. Physiol.-Endocrinol. Metab. 1992, 262, E911–E918. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Lee, J.L.; Dreyer, H.C.; Dhanani, S.; Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Sheffield-Moore, M.; Rasmussen, B.B.; Volpi, E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J. Clin. Endocrinol. Metab. 2010, 95, 3848–3857. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Khaled, A.R. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr. Med. Chem. 2012, 19, 3748–3762. [Google Scholar] [CrossRef] [PubMed]
- Everman, S.; Meyer, C.; Tran, L.; Hoffman, N.; Carroll, C.C.; Dedmon, W.L.; Katsanos, C.S. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E671–E677. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.D.; Bickerstaff, G.F.; Baker, J.S. Interactions of cortisol, testosterone, and resistance training: Influence of circadian rhythms. Chronobiol. Int. 2010, 27, 675–705. [Google Scholar] [CrossRef]
- Sung, C.C.; Liao, M.T.; Lu, K.C.; Wu, C.C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 2012, 634195. [Google Scholar] [CrossRef]
- Luu, T.M.; Katz, S.L.; Leeson, P.; Thébaud, B.; Nuyt, A.M. Preterm birth: Risk factor for early-onset chronic diseases. Can. Med Assoc. J. 2016, 188, 736–746. [Google Scholar] [CrossRef]
- Christiansen, L.I.; Holmqvist, B.; Pan, X.; Holgersen, K.; Lindholm, S.E.; Henriksen, N.L.; Burrin, D.G.; Ley, D.; Thymann, T.; Sangild, P.T. Insulin-like growth factor-1 supplementation promotes brain maturation in preterm pigs. eNeuro 2023, 10, 1–15. [Google Scholar] [CrossRef]
- Cutfield, W.S.; Regan, F.A.; Jackson, W.E.; Jefferies, C.A.; Robinson, E.M.; Harris, M.; Hofman, P.L. The endocrine consequences for very low birth weight premature infants. Growth Horm. IGF Res. 2004, 14, 130–135. [Google Scholar] [CrossRef]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Löfqvist, C.; Van Marter, L.; Van Weissenbruch, M.; Ramenghi, L.A.; Beardsall, K.; Dunger, D. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016, 105, 576–586. [Google Scholar] [CrossRef]
- Kwinta, P.; Klimek, M.; Wójcik, M.; Grudzień, A.; Drozdz, D.; Pietrzyk, J.J. Insulin-like growth factor-1 (IGF-1) serum concentration among 7-year-old extremely low birth weight children--an indicator of growth problems. J. Pediatr. Endocrinol. Metab. 2011, 24, 651–657. [Google Scholar] [CrossRef]
- Verkauskiene, R.; Jaquet, D.; Deghmoun, S.; Chevenne, D.; Czernichow, P.; Lévy-Marchal, C. Smallness for gestational age is associated with persistent change in insulin-like growth factor I (IGF-I) and the ratio of IGF-I/IGF-binding protein-3 in adulthood. J. Clin. Endocrinol. Metab. 2005, 90, 5672–5676. [Google Scholar] [CrossRef]
- Zeitlin, J.; Ancel, P.Y.; Saurel-Cubizolles, M.J.; Papiernik, E. The relationship between intrauterine growth restriction and preterm delivery: An empirical approach using data from a European case-control study. BJOG 2000, 107, 750–758. [Google Scholar] [CrossRef]
- Fu, Q.; Yu, X.; Callaway, C.W.; Lane, R.H.; McKnight, R.A. Epigenetics: Intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. FASEB J. 2009, 23, 2438–2449. [Google Scholar] [CrossRef]
- Kantake, M.; Ikeda, N.; Nakaoka, H.; Ohkawa, N.; Tanaka, T.; Miyabayashi, K.; Shoji, H.; Shimizu, T. IGF1 gene is epigenetically activated in preterm infants with intrauterine growth restriction. Clin. Epigenetics 2020, 12, 108. [Google Scholar] [CrossRef]
- Deprez, A.; Orfi, Z.; Radu, A.; He, Y.; Ravizzoni Dartora, D.; Dort, J.; Dumont, N.A.; Nuyt, A.M. Transient neonatal exposure to hyperoxia, an experimental model of preterm birth, leads to skeletal muscle atrophy and fiber type switching. Clin. Sci. 2021, 135, 2589–2605. [Google Scholar] [CrossRef]
- Dong, G.; Moparthy, C.; Thome, T.; Kim, K.; Yue, F.; Ryan, T.E. IGF-1 therapy improves muscle size and function in experimental peripheral arterial disease. Basic. Transl. Sci. 2023, 8, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Cuffe, S.A.; Plant, D.R.; Gregorevic, P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul. Disord. 2001, 11, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.B.; Micmacher, E.; Biesek, S.; Assumpção, R.; Redorat, R.; Veloso, U.; Vaisman, M.; Farinatti, P.T.; Conceição, F. Effects of Growth Hormone Administration on Muscle Strength in Men over 50 Years Old. Int. J. Endocrinol. 2013, 2013, 942030. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Pruitt, L.; Reim, J.; Hintz, R.L.; Butterfield, G.; Hoffman, A.R.; Marcus, R. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J. Clin. Endocrinol. Metab. 1994, 79, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhonggui, X.; Ping, Z.; Jian, K.; Feimin, S.; Zeyuan, X. The growth rates and influencing factors of preterm and full-term infants: A birth cohort study. Medicine 2022, 101, e30262. [Google Scholar] [CrossRef]
- Salas, A.A.; Jerome, M.; Finck, A.; Razzaghy, J.; Chandler-Laney, P.; Carlo, W.A. Body composition of extremely preterm infants fed protein-enriched, fortified milk: A randomized trial. Pediatr. Res. 2022, 91, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Valentine, C.J.; Fernandez, S.; Rogers, L.K.; Gulati, P.; Hayes, J.; Lore, P.; Puthoff, T.; Dumm, M.; Jones, A.; Collins, K.; et al. Early amino-acid administration improves preterm infant weight. J. Perinatol. 2009, 29, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Maggio, L.; Cota, F.; Gallini, F.; Lauriola, V.; Zecca, C.; Romagnoli, C. Effects of high versus standard early protein intake on growth of extremely low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 124–129. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Bass, K.D.; Thureen, P.J. Achieving positive protein balance in the immediate postoperative period in neonates undergoing abdominal surgery. J. Pediatr. 2008, 152, 63–67. [Google Scholar] [CrossRef]
- Tonkin, E.L.; Collins, C.T.; Miller, J. Protein Intake and Growth in Preterm Infants: A Systematic Review. Glob. Pediatr. Health 2014, 1, 2333794X14554698. [Google Scholar] [CrossRef] [PubMed]
- Withers, R.T.; LaForgia, J.; Pillans, R.K.; Shipp, N.J.; Chatterton, B.E.; Schultz, C.G.; Leaney, F.; Brage, S.; Ekelund, U.; Brage, N.; et al. Comparisons of two-, three-, and four-compartment models of body composition analysis in men and women. J. Appl. Physiol. 1998, 85, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.; Embleton, N.; Rigo, J.; Carrie, A.; Haschke, F.; Ziegler, E. High protein pre-term infant formula: Effect on nutrient balance, metabolic status and growth. Pediatr. Res. 2006, 59, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Rudar, M.; Suryawan, A.; Nguyen, H.V.; Chacko, S.K.; Vonderohe, C.; Stoll, B.; Burrin, D.G.; Fiorotto, M.L.; Davis, T.A. Regulation of skeletal muscle protein synthesis in the preterm pig by intermittent leucine pulses during continuous parenteral feeding. JPEN J. Parenter. Enter. Nutr. 2023, 47, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rudar, M.; Naberhuis, J.K.; Suryawan, A.; Nguyen, H.V.; Stoll, B.; Style, C.C.; Verla, M.A.; Olutoye, O.O.; Burrin, D.G.; Fiorotto, M.L.; et al. Prematurity blunts the insulin- and amino acid-induced stimulation of translation initiation and protein synthesis in skeletal muscle of neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E551–E565. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yang, Q.; Wang, H.; Melick, C.H.; Navlani, R.; Frank, A.R.; Jewell, J.L. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020, 295, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Casirati, A.; Somaschini, A.; Perrone, M.; Vandoni, G.; Sebastiani, F.; Montagna, E.; Somaschini, M.; Caccialanza, R. Preterm birth and metabolic implications on later life: A narrative review focused on body composition. Front. Nutr. 2022, 9, 978271. [Google Scholar] [CrossRef] [PubMed]
- Obst, S.; Herz, J.; Alejandre Alcazar, M.A.; Endesfelder, S.; Möbius, M.A.; Rüdiger, M.; Felderhoff-Müser, U.; Bendix, I. Perinatal Hyperoxia and Developmental Consequences on the Lung-Brain Axis. Oxid. Med. Cell Longev. 2022, 2022, 5784146. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Mourkioti, F.; Rosenthal, N. NF-kappaB signaling in skeletal muscle: Prospects for intervention in muscle diseases. J. Mol. Med. 2008, 86, 747–759. [Google Scholar] [CrossRef]
- Peris-Moreno, D.; Taillandier, D.; Polge, C. MuRF1/TRIM63, Master Regulator of Muscle Mass. Int. J. Mol. Sci. 2020, 21, 6663. [Google Scholar] [CrossRef]
- Kumari, S.; Barton, G.P.; Goss, K.N. Increased mitochondrial oxygen consumption in adult survivors of preterm birth. Pediatr. Res. 2021, 90, 1147–1152. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose-Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- Goss, K.N.; Kumari, S.; Tetri, L.H.; Barton, G.; Braun, R.K.; Hacker, T.A.; Eldridge, M.W. Postnatal hyperoxia exposure durably impairs right ventricular function and mitochondrial biogenesis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 609–619. [Google Scholar] [CrossRef]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Neonatal Network; German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. In Proceedings of the Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 451–468. [Google Scholar]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Costill, D.L.; Daniels, J.; Evans, W.; Fink, W.; Krahenbuhl, G.; Saltin, B.; Mitchell, E.A.; Martin, N.R.W.; Bailey, S.J.; Ferguson, R.A.; et al. Skeletal muscle enzymes and fiber composition in male and female track athletes. J. Appl. Physiol. 1976, 40, 149–154. [Google Scholar] [CrossRef]
- Hall, E.C.; Lysenko, E.A.; Semenova, E.A.; Borisov, O.V.; Andryushchenko, O.N.; Andryushchenko, L.B.; Vepkhvadze, T.F.; Lednev, E.M.; Zmijewski, P.; Popov, D.V. Prediction of muscle fiber composition using multiple repetition testing. Biol. Sport. 2021, 38, 277–283. [Google Scholar] [CrossRef]
- Dong, H.; Tsai, S.-Y. Mitochondrial properties in skeletal muscle fiber. Cells 2023, 12, 2183. [Google Scholar] [CrossRef]
- Anderson, E.J.; Neufer, P.D. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am. J. Physiol.-Cell Physiol. 2006, 290, C844–C851. [Google Scholar] [CrossRef]
- Wang, D.T.; Yin, Y.; Yang, Y.J.; Lv, P.J.; Shi, Y.; Lu, L.; Wei, L.B. Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, C.Y.; Zhang, J.T.; Gu, L.M.; Xu, G.T.; Zhang, J.F. Epigenetic modifications and metabolic memory in diabetic retinopathy: Beyond the surface. Neural Regen. Res. 2023, 18, 1441–1449. [Google Scholar] [CrossRef]
- Ling, C.; Groop, L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes 2009, 58, 2718–2725. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Sedlackova, L.; Korolchuk, V.I. The crosstalk of NAD, ROS and autophagy in cellular health and ageing. Biogerontology 2020, 21, 381–397. [Google Scholar] [CrossRef]
- Wilson, N.; Kataura, T.; Korsgen, M.E.; Sun, C.; Sarkar, S.; Korolchuk, V.I. The autophagy-NAD axis in longevity and disease. Trends Cell Biol. 2023, 33, 788–802. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [CrossRef]
- Lin, S.J.; Ford, E.; Haigis, M.; Liszt, G.; Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes. Dev. 2004, 18, 12–16. [Google Scholar] [CrossRef]
- Schmidt, M.T.; Smith, B.C.; Jackson, M.D.; Denu, J.M. Coenzyme specificity of Sir2 protein deacetylases: Implications for physiological regulation. J. Biol. Chem. 2004, 279, 40122–40129. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 2010, 10, 12–31. [Google Scholar] [CrossRef]
- Eisenberg, T.; Schroeder, S.; Büttner, S.; Carmona-Gutierrez, D.; Pendl, T.; Andryushkova, A.; Mariño, G.; Pietrocola, F.; Harger, A.; Zimmermann, A. A histone point mutation that switches on autophagy. Autophagy 2014, 10, 1143–1145. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Schmidt, O.; Pfanner, N.; Meisinger, C. Mitochondrial protein import: From proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 2010, 11, 655–667. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Fryer, A.A.; Emes, R.D.; Ismail, K.M.; Haworth, K.E.; Mein, C.; Carroll, W.D.; Farrell, W.E. Quantitative, high-resolution epigenetic profiling of CpG loci identifies associations with cord blood plasma homocysteine and birth weight in humans. Epigenetics 2011, 6, 86–94. [Google Scholar] [CrossRef]
- Burris, H.H.; Rifas-Shiman, S.L.; Baccarelli, A.; Tarantini, L.; Boeke, C.E.; Kleinman, K.; Litonjua, A.A.; Rich-Edwards, J.W.; Gillman, M.W. Associations of long interspersed nuclear element-1 DNA methylation with preterm birth in a prospective cohort study. J. Dev. Orig. Health Dis. 2012, 3, 173–181. [Google Scholar] [CrossRef]
- Relton, C.L.; Groom, A.; St Pourcain, B.; Sayers, A.E.; Swan, D.C.; Embleton, N.D.; Pearce, M.S.; Ring, S.M.; Northstone, K.; Tobias, J.H.; et al. DNA methylation patterns in cord blood DNA and body size in childhood. PLoS ONE 2012, 7, e31821. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobson, N.L.; Levitt, D.E.; Luk, H.Y.; Vellers, H.L. Adverse Skeletal Muscle Adaptations in Individuals Born Preterm—A Comprehensive Review. Curr. Issues Mol. Biol. 2024, 46, 4551-4564. https://doi.org/10.3390/cimb46050276
Dobson NL, Levitt DE, Luk HY, Vellers HL. Adverse Skeletal Muscle Adaptations in Individuals Born Preterm—A Comprehensive Review. Current Issues in Molecular Biology. 2024; 46(5):4551-4564. https://doi.org/10.3390/cimb46050276
Chicago/Turabian StyleDobson, Nick L., Danielle E. Levitt, Hui Ying Luk, and Heather L. Vellers. 2024. "Adverse Skeletal Muscle Adaptations in Individuals Born Preterm—A Comprehensive Review" Current Issues in Molecular Biology 46, no. 5: 4551-4564. https://doi.org/10.3390/cimb46050276





