Long-Term Alterations in Motor Skills, Neurogenesis and Astrocyte Numbers following Transient Cerebral Ischemia in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
2.3. Chemicals and Antibodies
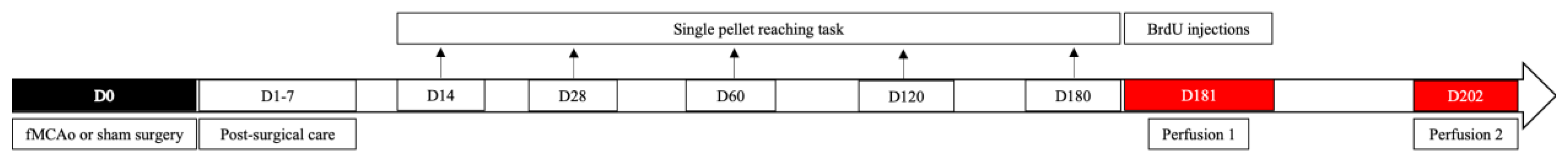
2.4. Filament Middle Cerebral Artery Occlusion (fMCAo) Model
2.5. Post-Operative Care
2.6. Single Pellet Reaching Task
2.7. BrdU Injections
2.8. Brain Tissue Preparation
2.9. Immunofluorescence
2.10. Image Acquisition and Processing
2.11. Statistical Analysis
- Sham ipsi against fMCAo ipsi group;
- Sham contra against fMCAo contra group;
- Sham ipsi against Sham contra group;
- fMCAo ipsi against fMCAo contra group.
3. Results
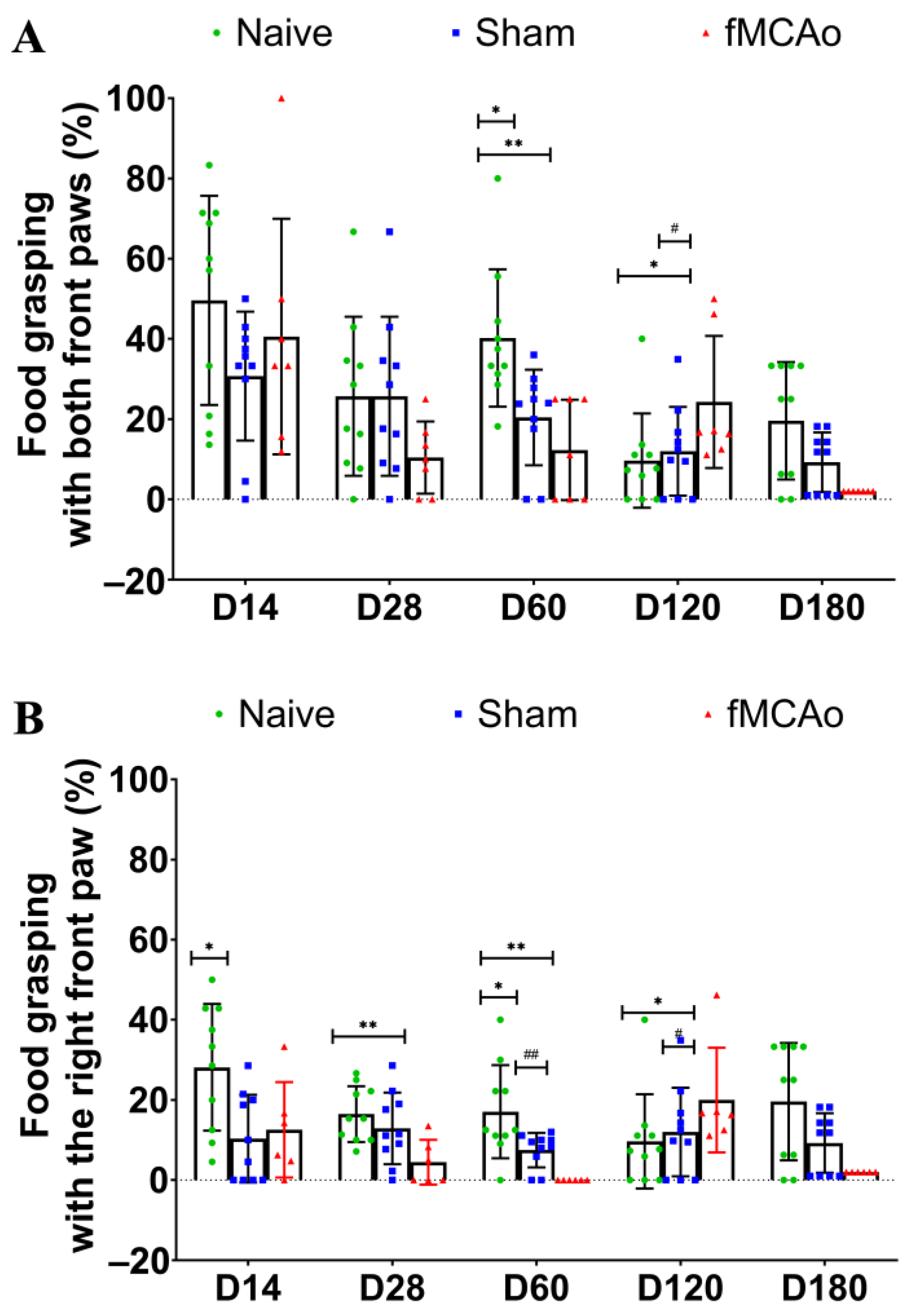
3.1. Impaired Reaching Ability of fMCAo Mice
3.2. Altered BrdU+/DCX+ Cell Numbers in the SVZ Region
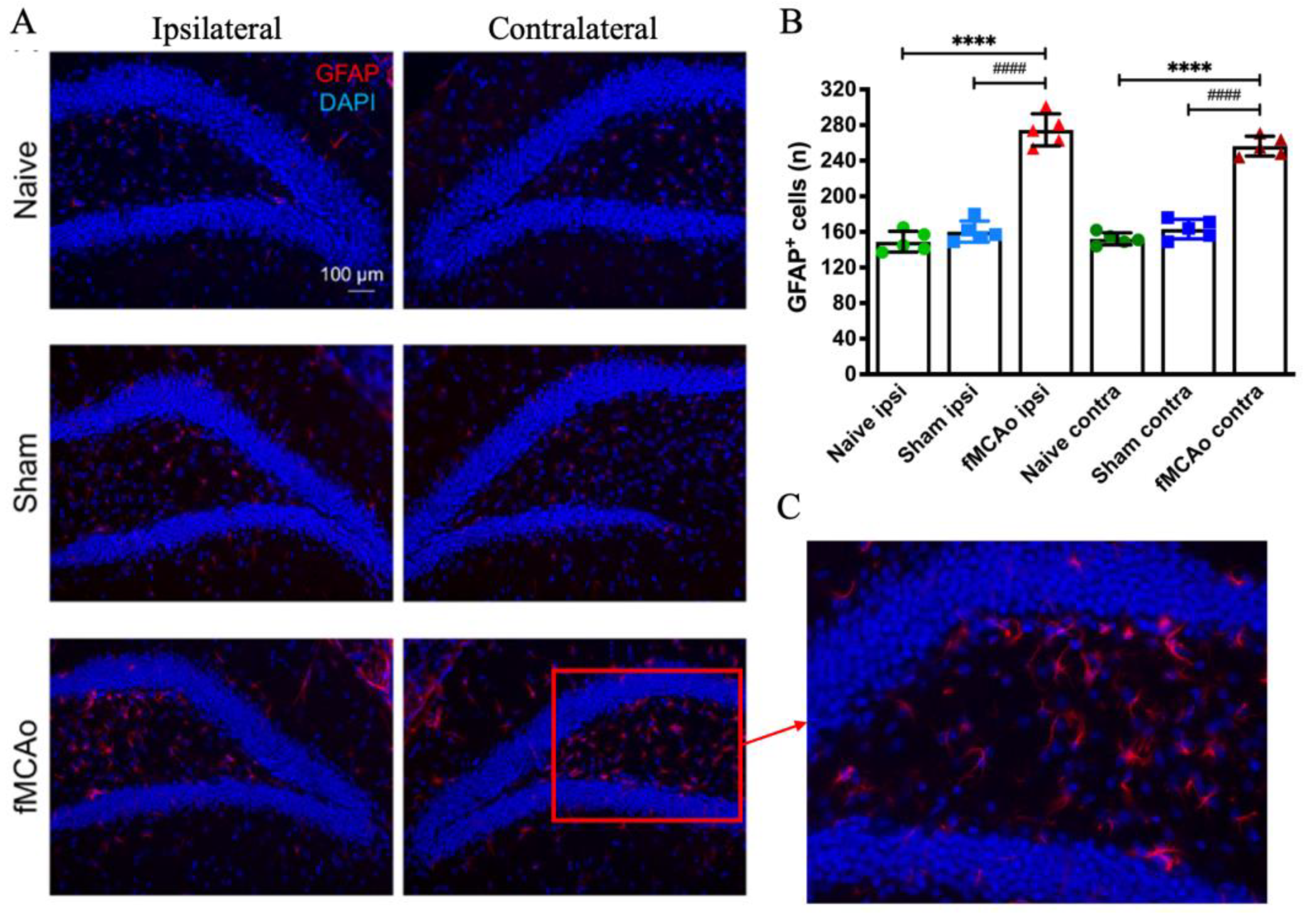
3.3. Changes in GFAP+ Cells in the Hippocampal DG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holloway, P.M.; Gavins, F.N.E. Modeling Ischemic Stroke In Vitro: Status Quo and Future Perspectives. Stroke 2016, 47, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Auriel, E.; Bornstein, N.M. Neuroprotection in acute ischemic stroke—Current status. J. Cell Mol. Med. 2010, 14, 2200–2202. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Wlodarczyk, L.; Szelenberger, R.; Cichon, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. Biomarkers of angiogenesis and neuroplasticity as promising clinical tools for stroke recovery evaluation. Int. J. Mol. Sci. 2021, 22, 3949. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.L.; Carvalho, G.A.; Freitas, E.M.M.; Chiareli, R.A.; Barbosa, T.G.; Di Araújo, A.G.P.; Nogueira, Y.L.; Ribeiro, R.I.; Parreira, R.C.; Vieira, M.S.; et al. The role of neurogenesis in neurorepair after ischemic stroke. Semin. Cell Dev. Biol. 2019, 95, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Wang, B.; Zhuge, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient Expression of Doublecortin during Adult Neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, K. Current perspectives on the link between neuroinflammation and neurogenesis. Metab. Brain Dis. 2015, 30, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.O.; Zhu, W.; Wang, Y.; Shen, J.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef]
- Kojima, T.; Hirota, Y.; Ema, M.; Takahashi, S.; Miyoshi, I.; Okano, H.; Sawamoto, K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 2010, 28, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.-W.; Chang, D.; Jenkins, A.; Bensoussan, A.; Kiat, H. Angiogenesis in Ischemic Stroke and Angiogenic Effects of Chinese Herbal Medicine. J. Clin. Med. 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D. Estimates of the Prevalence of Acute Stroke Impairments and Disability in a Multiethnic Population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lim, S.H.; Kim, K.H.; Kim, K.J.; Kim, Y.R.; Chang, W.N.; Yeom, J.W.; Kim, Y.D.; Hwang, B.Y. Six-month functional recovery of stroke patients. Int. J. Rehabil. Res. 2015, 38, 173–180. [Google Scholar] [CrossRef]
- Nichols-Larsen, D.S.; Clark, P.C.; Zeringue, A.; Greenspan, A.; Blanton, S. Factors Influencing Stroke Survivors’ Quality of Life during Subacute Recovery. Stroke 2005, 36, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Foroud, A.; Whishaw, I.Q. Changes in the kinematic structure and non-kinematic features of movements during skilled reaching after stroke: A Laban Movement Analysis in two case studies. J. Neurosci. Methods 2006, 158, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.; Uswatte, G.; Mark, V.W. The functional significance of cortical reorganization and the parallel development of CI therapy. Front. Hum. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Sharp, F.R. Improving the translation of animal ischemic stroke studies to humans. Metab. Brain Dis. 2015, 30, 461–467. [Google Scholar] [CrossRef]
- Linden, J.; Van de Beeck, L.; Plumier, J.C.; Ferrara, A. Procedural learning as a measure of functional impairment in a mouse model of ischemic stroke. Behav. Brain Res. 2016, 307, 35–45. [Google Scholar] [CrossRef]
- Rosell, A.; Agin, V.; Rahman, M.; Morancho, A.; Ali, C.; Koistinaho, J.; Wang, X.; Vivien, D.; Schwaninger, M.; Montaner, J. Distal Occlusion of the Middle Cerebral Artery in Mice: Are We Ready to Assess Long-Term Functional Outcome? Transl. Stroke Res. 2013, 4, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Yao, Y. Behavioral tests in rodent models of stroke. Brain Hemorrhages 2020, 1, 171–184. [Google Scholar] [CrossRef]
- Klein, A.; Sacrey, L.-A.R.; Whishaw, I.Q.; Dunnett, S.B. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci. Biobehav. Rev. 2012, 36, 1030–1042. [Google Scholar] [CrossRef]
- Bu, F.; Min, J.-W.; Munshi, Y.; Lai, Y.-J.; Qi, L.; Urayama, A.; McCullough, L.D.; Li, J. Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice. Exp. Neurol. 2019, 322, 113059. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Gilmore, A.; Zuo, Y. Study Motor Skill Learning by Single-pellet Reaching Tasks in Mice. J. Vis. Exp. 2014, 85, e51238. [Google Scholar] [CrossRef]
- Pilipenko, V.; Dzirkale, Z.; Rozkalne, R.; Upite, J.; Hellal, F.; Plesnila, N.; Jansone, B. Focal Cerebral Ischemia Induces Global Subacute Changes in the Number of Neuroblasts and Neurons and the Angiogenic Factor Density in Mice. Medicina (B Aires) 2023, 59, 2168. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates BT—123Library, 6th ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Farr, T.D.; Whishaw, I.Q. Quantitative and Qualitative Impairments in Skilled Reaching in the Mouse (Mus musculus) After a Focal Motor Cortex Stroke. Stroke 2002, 33, 1869–1875. [Google Scholar] [CrossRef]
- MacLellan, C.L.; Gyawali, S.; Colbourne, F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav. Brain Res. 2006, 175, 82–89. [Google Scholar] [CrossRef]
- Balkaya, M.; Kröber, J.M.; Rex, A.; Endres, M. Assessing Post-Stroke Behavior in Mouse Models of Focal Ischemia. J. Cereb. Blood Flow. Metab. 2013, 33, 330–338. [Google Scholar] [CrossRef]
- Girard, S.; Murray, K.N.; Rothwell, N.J.; Metz, G.A.S.; Allan, S.M. Long-term functional recovery and compensation after cerebral ischemia in rats. Behav. Brain Res. 2014, 270, 18–28. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Ruhoff, A.M.; Fath, T.; Jones, N.M. Hypoxic postconditioning enhances functional recovery following endothelin-1 induced middle cerebral artery occlusion in conscious rats. Exp. Neurol. 2018, 306, 177–189. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Lee, K.-H.; Jeon, H.-E.; Hwang, B.-H.; Beak, J.-W.; Joa, K.-L.; Kang, J.-H. Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion. Int. J. Mol. Sci. 2020, 21, 5842. [Google Scholar] [CrossRef]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent Production of Neurons from Adult Brain Stem Cells During Recovery after Stroke. Stem Cells 2006, 24, 739–747. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.P.; Ogle, M.E.; Ding, X.S.; Wei, L. Enhanced neurogenesis and cell migration following focal ischemia and peripheral stimulation in mice. Dev. Neurobiol. 2008, 68, 1474–1486. [Google Scholar] [CrossRef]
- Bao, Y.; Qin, L.; Kim, E.; Bhosle, S.; Guo, H.; Febbraio, M.; Haskew-Layton, R.E.; Ratan, R.; Cho, S. CD36 is Involved in Astrocyte Activation and Astroglial Scar Formation. J. Cereb. Blood Flow. Metab. 2012, 32, 1567–1577. [Google Scholar] [CrossRef]
- Mestriner, R.G.; Saur, L.; Bagatini, P.B.; Baptista, P.P.A.; Vaz, S.P.; Ferreira, K.; Machado, S.A.; Xavier, L.L.; Netto, C.A. Astrocyte morphology after ischemic and hemorrhagic experimental stroke has no influence on the different recovery patterns. Behav. Brain Res. 2015, 278, 257–261. [Google Scholar] [CrossRef]
- Alaverdashvili, M.; Whishaw, I.Q. A behavioral method for identifying recovery and compensation: Hand use in a preclinical stroke model using the single pellet reaching task. Neurosci. Biobehav. Rev. 2013, 37, 950–967. [Google Scholar] [CrossRef]
- Tariq, M.B.; Lee, J.; McCullough, L.D. Sex differences in the inflammatory response to stroke. Semin. Immunopathol. 2023, 45, 295–313. [Google Scholar] [CrossRef]

| Antibody | Manufacturer | Concentration | Cat. No. | RRID |
|---|---|---|---|---|
| Mouse anti-BrdU | Thermo Fisher Scientific (Waltham, MA, USA) | 1:4000 | MA3-071 | AB_10986341 |
| Goat anti-mouse IgG2A (AlexaFluor® 488-conjugated) | 1:1000 | A21131 | AB_2535771 | |
| Rabbit anti-DCX | Abcam (Cambridge, UK) | 1:50 | ab207175 | AB_2894710 |
| Rabbit anti-GFAP | 1:250 | ab68428 | AB_1209224 | |
| Goat anti-rabbit IgG (AlexaFluor® 594-conjugated) | 1:1000 | ab150084 | AB_2734147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilipenko, V.; Upite, J.; Revina, B.L.; Jansone, B. Long-Term Alterations in Motor Skills, Neurogenesis and Astrocyte Numbers following Transient Cerebral Ischemia in Mice. Medicina 2024, 60, 658. https://doi.org/10.3390/medicina60040658
Pilipenko V, Upite J, Revina BL, Jansone B. Long-Term Alterations in Motor Skills, Neurogenesis and Astrocyte Numbers following Transient Cerebral Ischemia in Mice. Medicina. 2024; 60(4):658. https://doi.org/10.3390/medicina60040658
Chicago/Turabian StylePilipenko, Vladimirs, Jolanta Upite, Beatrise Luize Revina, and Baiba Jansone. 2024. "Long-Term Alterations in Motor Skills, Neurogenesis and Astrocyte Numbers following Transient Cerebral Ischemia in Mice" Medicina 60, no. 4: 658. https://doi.org/10.3390/medicina60040658





