The Effect of Recruitment Maneuver on Static Lung Compliance in Patients Undergoing General Anesthesia for Laparoscopic Cholecystectomy: A Single-Centre Prospective Clinical Intervention Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Anesthesia Technique
2.3. Recruitment Maneuver
2.4. Hemodynamic and Respiratory Monitoring
2.5. Statistical Analysis
3. Results
3.1. Anthropometric and Demographic Characteristics of the Participants
3.2. Parameters of Respiratory Mechanics
3.3. Gas Exchange
3.4. Hemodynamic Parameters
4. Discussion
4.1. Respiratory Mechanics
4.2. Blood Gas Analyses
4.3. Hemodynamic Changes
4.4. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019, 123, 898–913. [Google Scholar] [CrossRef]
- Hess, D.R. Respiratory mechanics in mechanically ventilated patients. Respir. Care 2014, 59, 1773–1794. [Google Scholar] [CrossRef]
- Banks, J.A. Efficacy of Alveolar Recruitment Maneuvers in the Adult Obese Patient Undergoing General Anesthesia: A Systematic Review of the Literature. Master’s Thesis, Rhode Island University, Kingston, RI, USA, 2018. Available online: https://digitalcommons.ric.edu/etd/291 (accessed on 12 December 2023).
- Li, X.; Ni, Z.-L.; Wang, J.; Liu, X.-C.; Guan, H.-L.; Dai, M.-S.; Gao, X.; Zhou, Y.; Hu, X.-Y.; Sun, X.; et al. Effects of individualized positive end-expiratory pressure combined with recruitment maneuver on intraoperative ventilation during abdominal surgery: A systematic review and network meta-analysis of randomized controlled trials. J. Anesth. 2022, 36, 303–315. [Google Scholar] [CrossRef]
- Elokada, S.A.; Farag, H.M. Preemptive Alveolar Recruitment Maneuver Followed by PEEP in Obese Patients Undergoing Laparoscopic Gastric Banding. Does it make a Difference? A Randomized Controlled Clinical Study. Open Anesth. J. 2019, 13, 31–39. [Google Scholar] [CrossRef]
- Wynn-Hebden, A.; Bouch, D.C. Anaesthesia for the obese patient. BJA Educ. 2020, 20, 388–395. [Google Scholar] [CrossRef]
- Bluth, T.; Neto, A.S.; Schultz, M.J.; Pelosi, P.; de Abreu, M.G. Effect of Intraoperative High Positive End-Expiratory Pressure (PEEP) with Recruitment Maneuvers vs Low PEEP on Postoperative Pulmonary Complications in Obese Patients: A Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2019, 321, 2292–2305. [Google Scholar]
- Tomescu, D.R.; Popescu, M.; Dima, S.O.; Bacalbașa, N.; Bubenek-Turconi, Ș. Obesity is associated with decreased lung compliance and hypercapnia during robotic assisted surgery. J. Clin. Monit. Comput. 2017, 31, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kock, K.S.; Maurici, R. Respiratory mechanics, ventilator-associated pneumonia and outcomes in intensive care unit. World J. Crit. Care Med. 2018, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Grieco, D.L.; Beloncle, F.; Chen, G.-Q.; Tiribelli, N.; Madotto, F.; Fredes, S.; Lu, C.; Antonelli, M.; Mercat, A.; et al. Partition of respiratory mechanics in patients with acute respiratory distress syndrome and association with outcome: A multicentre clinical study. Intensive Care Med. 2022, 48, 888–898. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Lucangelo, U.; Bernabé, F.; Blanch, L. Respiratory mechanics derived from signals in the ventilator circuit. Respir. Care 2005, 50, 55–65. [Google Scholar] [PubMed]
- Meier, A.; Sell, R.E.; Malhotra, A. Driving Pressure for Ventilation of Patients with Acute Respiratory Distress Syndrome. Anesthesiology 2020, 132, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.; Engberg, G.; Sporre, B.; Kviele, L.; Rothen, H.U.; Wegenius, G.; Nylund, U.; Hedenstierna, G. Volumetric analysis of aeration in the lungs during general anaesthesia. Br. J. Anaesth. 1996, 76, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Whalen, F.X.; Gajic, O.; Thompson, G.B.; Kendrick, M.L.; Que, F.L.; Williams, B.A.; Joyner, M.J.; Hubmayr, R.D.; Warner, D.O.; Sprung, J. The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth. Analg. 2006, 102, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Tredici, S.; Pedoto, A.; Lissoni, A. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998, 87, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Steier, J.; Jolley, C.J.; Seymour, J.; Roughton, M.; Polkey, M.I.; Moxham, J. Neural respiratory drive in obesity. Thorax 2009, 64, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Foti, G.; Cereda, M.; Vicardi, P.; Gattinoni, L. Effects of carbon dioxide insufflation for laparoscopic cholecystectomy on the respiratory system. Anaesthesia 1996, 51, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Sprung, J.; Whalley, D.G.; Falcone, T.; Warner, D.O.; Hubmayr, R.D.; Hammel, J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth. Analg. 2002, 94, 1345–1350. [Google Scholar] [CrossRef]
- Talab, H.F.; Zabani, I.A.; Abdelrahman, H.S.; Bukhari, W.L.; Mamoun, I.; Ashour, M.A.; Bin Sadeq, B.; El Sayed, S.I. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth. Analg. 2009, 109, 1511–1516. [Google Scholar] [CrossRef]
- Andersson, L.E.; Bååth, M.; Thörne, A.; Aspelin, P.; Odeberg-Wernerman, S. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology 2005, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.C.; Brandstetter, R.D.; Brensilver, J.M.; Jung, L.D. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest 1996, 110, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.-M.; Pelosi, P.; Chanques, G.; Kwiatkoskwi, F.; Jaber, S.; Bazin, J.-E. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 2010, 113, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wei, W.; Yang, K.; Yang, Y.; Pan, Y.; Wei, J.; Yao, S.; Xia, H. Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5841. [Google Scholar] [CrossRef] [PubMed]
- Almarakbi, W.A.; Fawzi, H.M.; Alhashemi, J.A. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. Br. J. Anaesth. 2009, 102, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Hartland, B.L.; Newell, T.J.; Damico, N. Alveolar recruitment maneuvers under general anesthesia: A systematic review of the literature. Respir. Care 2015, 60, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Marini, J.J.; Pesenti, A.; Quintel, M.; Mancebo, J.; Brochard, L. The “baby lung” became an adult. Intensive Care Med. 2016, 42, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Del Sorbo, L.; Grieco, D.L.; Junhasavasdikul, D.; Rittayamai, N.; Soliman, I.; Sklar, M.C.; Rauseo, M.; Ferguson, N.D.; Fan, E.; et al. Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Carlesso, E.; Cressoni, M. Selecting the ‘right’ positive end-expiratory pressure level. Curr. Opin. Crit. Care 2015, 21, 50–57. [Google Scholar] [CrossRef]
- Mazzinari, G.; Diaz-Cambronero, O.; Alonso-Iñigo, J.M.; Garcia-Gregorio, N.; Ayas-Montero, B.; Ibañez, J.L.; Neto, A.S.; Ball, L.; de Abreu, M.G.; Pelosi, P.; et al. Intraabdominal Pressure Targeted Positive End-expiratory Pressure during Laparoscopic Surgery: An Open-label, Nonrandomized, Crossover, Clinical Trial. Anesthesiology 2020, 132, 667–677. [Google Scholar] [CrossRef]
- Güldner, A.; Kiss, T.; Serpa Neto, A.; Hemmes, S.N.; Canet, J.; Spieth, P.M.; Rocco, P.R.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: A comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015, 123, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Hemmes, S.N.T.; Barbas, C.S.V.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; El-Tahan, M.R.; Al Ghamdi, A.A.; Günay, E.; et al. PROVE Network Investigators. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A meta-analysis of individual patient data. Lancet Respir. Med. 2016, 4, 272–280, Erratum in Lancet Respir. Med. 2016, 4, e34.. [Google Scholar] [CrossRef] [PubMed]
- del Portillo, I.P.; Vázquez, S.T.; Mendoza, J.B.; Moreno, R.V. Oxygen Therapy in Critical Care: A Double Edged Sword. Health 2014, 6, 2035–2046. [Google Scholar] [CrossRef]
- Pelosi, P.; Ravagnan, I.; Giurati, G.; Panigada, M.; Bottino, N.; Tredici, S.; Eccher, G.; Gattinoni, L. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology 1999, 91, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Myatra, S.N. Hemodynamic effects of alveolar recruitment maneuvres in the operating room: Proceed with caution. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Lovas, A.; Szakmány, T. Haemodynamic Effects of Lung Recruitment Manoeuvres. Biomed. Res. Int. 2015, 2015, 478970. [Google Scholar] [CrossRef] [PubMed]
- Biais, M.; Lanchon, R.; Sesay, M.; Le Gall, L.; Pereira, B.; Futier, E.; Nouette-Gaulain, K. Changes in Stroke Volume Induced by Lung Recruitment Maneuver Predict Fluid Responsiveness in Mechanically Ventilated Patients in the Operating Room. Anesthesiology 2017, 126, 260–267. [Google Scholar] [CrossRef]
- Simon, P.; Girrbach, F.; Petroff, D.; Schliewe, N.; Hempel, G.; Lange, M.; Bluth, T.; de Abreu, M.G.; Beda, A.; Schultz, M.J.; et al. Individualized versus Fixed Positive End-expiratory Pressure for Intraoperative Mechanical Ventilation in Obese Patients: A Secondary Analysis. Anesthesiology 2021, 134, 887–900. [Google Scholar] [CrossRef]
| Variable (Factor) | Normal-Weight (n = 33) | Pre-Obese and Obese Class I (n = 63) | p Value |
|---|---|---|---|
| Gender n (%) | 0.555 | ||
| Male | 13 (39.4) | 21 (33.3) | |
| Female | 20 (60.6) | 42 (66.7) | |
| Age | 46.91 ± 16.74 | 54.78 ± 13.21 | 0.013 |
| Height (cm) | 171.58 ± 9.01 | 168.00 ± 10.23 | 0.094 |
| Weight (kg) | 68.97 ± 9.45 | 83.70 ± 11.73 | <0.001 |
| BMI (kg/m2) | 23.33 ± 1.51 | 29.58 ± 2.53 | <0.001 |
| Neck circumference (cm) | 38.20 ± 9.59 | 39.79 ± 3.48 | 0.241 |
| Waist circumference (cm) | 82.52 ± 8.07 | 96.48 ± 10.35 | <0.001 |
| Hip circumference (cm) | 95.45 ± 5.89 | 106.78 ± 9.67 | <0.001 |
| Waist/hip circumference ratio | 0.86 ± 0.07 | 0.91 ± 0.07 | 0.011 |
| ASA class n (%) | 0.006 | ||
| ASA—1 | 11 (33,3) | 6 (9.5) | |
| ASA—2 | 19 (57.6) | 40 (63.5) | |
| ASA—3 | 3 (9.1) | 17 (27.0) |
| Time | Normal-Weight Group I BMI 18.5–24.9 (n = 33) | Pre-Obese and Obese Class I Group II BMI 25.0–34.9 (n = 63) | p-Value | |
|---|---|---|---|---|
| Ppeak (cmH2O) | T1 | 13.15 ± 2.24 | 15.63 ± 2.82 | <0.001 |
| T2 | 17.09 ± 2.54 | 20.08 ± 2.87 | <0.001 | |
| T3 | 18.70 ± 2.52 | 21.08 ± 2.44 | <0.001 | |
| T4 | 20.00 ± 2.06 | 21.98 ± 2.42 | <0.001 | |
| T5 | 17.55 ± 1.84 | 20.19 ± 2.36 | <0.001 | |
| T6 | 15.97 ± 1.99 | 17.79 ± 1.89 | <0.001 | |
| Pplat (cmH2O) | T1 | 10.15 ± 2.09 | 12.37 ± 2.55 | <0.001 |
| T2 | 14.00 ± 2.32 | 16.70 ± 2.61 | <0.001 | |
| T3 | 15.58 ± 2.42 | 17.63 ± 2.51 | <0.001 | |
| T4 | 17.30 ± 2.21 | 18.54 ± 2.24 | 0.008 | |
| T5 | 14.33 ± 1.87 | 16.02 ± 1.75 | <0.001 | |
| T6 | 13.52 ± 1.80 | 14.92 ± 1.80 | <0.001 | |
| Cdin (mL/cmH2O) | T1 | 60.09 ± 11.78 | 51.04 ± 11.95 | 0.001 |
| T2 | 41.86 ± 6.84 | 36.70 ± 7.13 | 0.001 | |
| T3 | 45.26 ± 8.14 | 39.03 ± 6.75 | <0.001 | |
| T4 | 46.50 ± 6.81 | 41.87 ± 7.49 | 0.004 | |
| T5 | 60.89 ± 12.31 | 50.49 ± 10.27 | <0.001 | |
| T6 | 68.56 ± 12.42 | 59.27 ± 11.24 | <0.001 | |
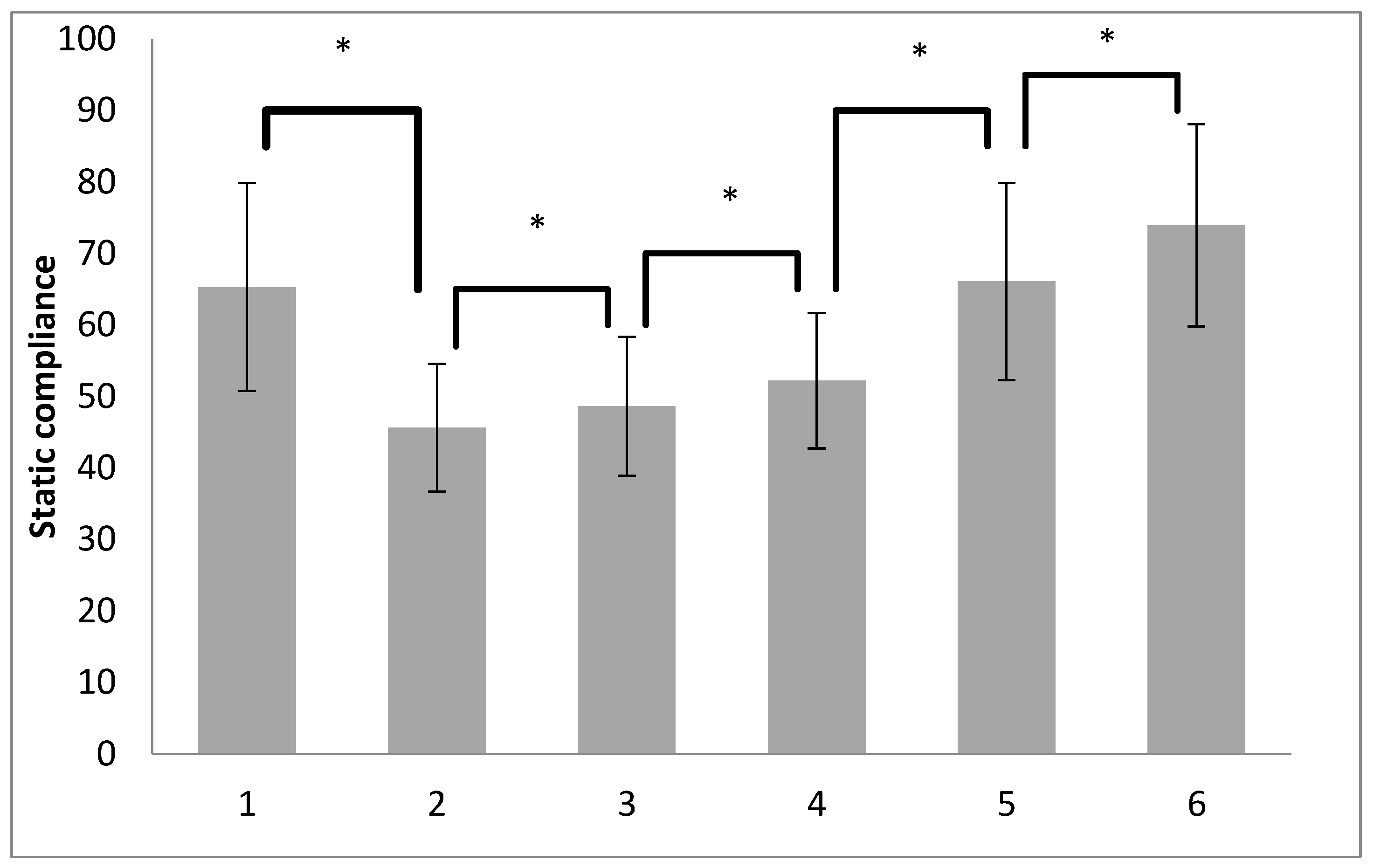
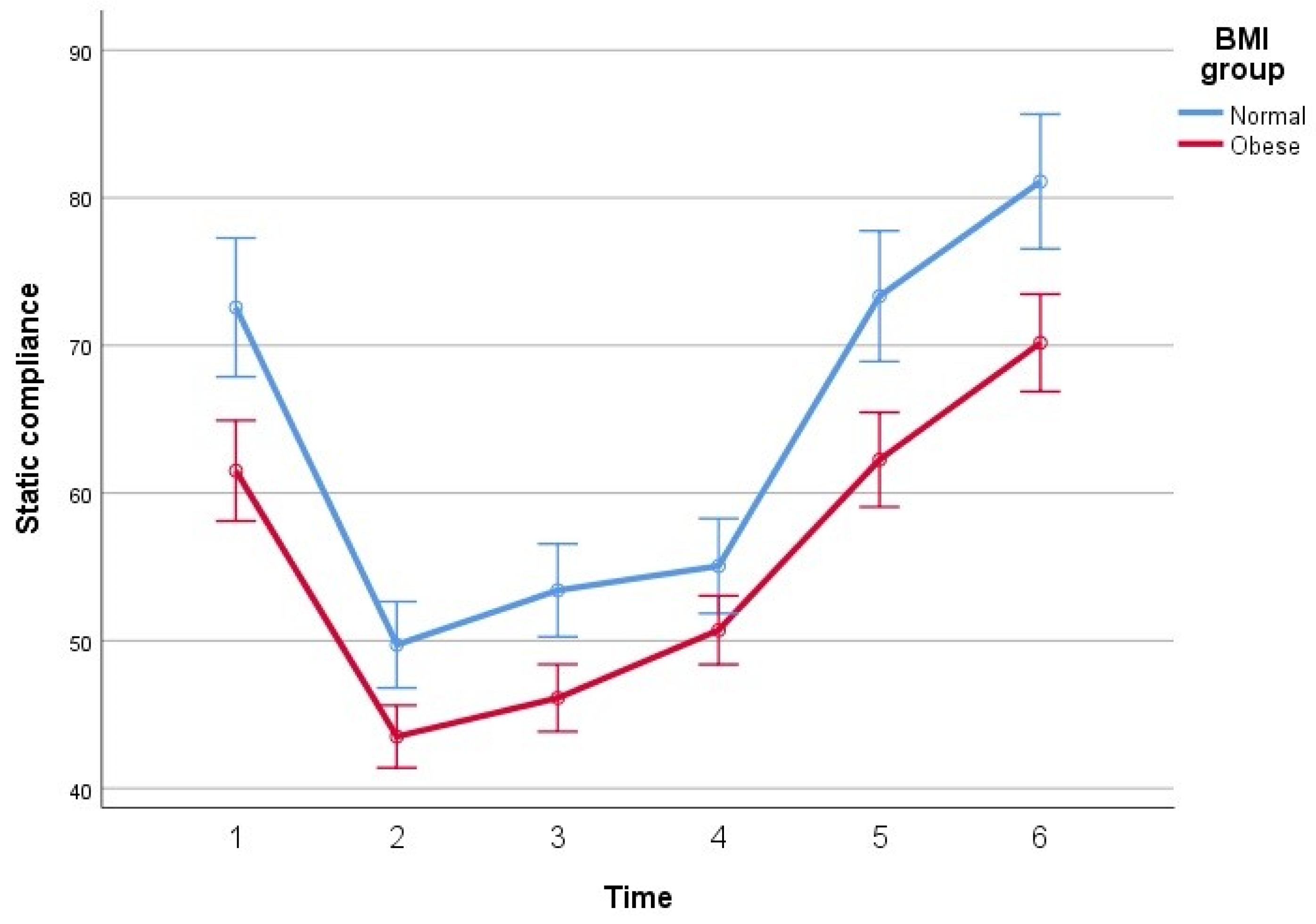
| Cstat (mL/cmH2O) | T1 | 72.58 ± 12.92 | 61.51 ± 13.95 | <0.001 |
| T2 | 49.73 ± 8.95 | 43.50 ± 8.20 | 0.001 | |
| T3 | 53.41 ± 11.10 | 46.12 ± 7.87 | <0.001 | |
| T4 | 55.06 ± 8.68 | 50.71 ± 9.60 | 0.032 | |
| T5 | 73.34 ± 13.51 | 62.26 ± 12.41 | <0.001 | |
| T6 | 81.10 ± 13.34 | 70.18 ± 13.12 | <0.001 | |
| DP (cmH2O) | T1 | 7.15 ± 2.09 | 9.37 ± 2.55 | <0.001 |
| T2 | 11.00 ± 2.32 | 13.70 ± 2.61 | <0.001 | |
| T3 | 10.58 ± 2.42 | 12.63 ± 2.51 | <0.001 | |
| T4 | 10.30 ± 2.21 | 11.54 ± 2.24 | 0.008 | |
| T5 | 7.33 ± 1.87 | 9.02 ± 1.75 | <0.001 | |
| T6 | 6.52 ± 1.80 | 7.92 ± 1.80 | <0.001 |
| Time Interval | Normal-Weight | Overweight and Obese |
|---|---|---|
| Δ2–Δ1 | 22.85 ± 8.09 | 18.00 ± 8.39 * |
| Δ3–Δ2 | 3.68 ± 5.35 | 2.62 ± 4.30 |
| Δ4–Δ3 | 1.65 ± 7.15 | 4.59 ± 4.76 |
| Δ5–Δ4 | 18.28 ± 7.40 | 11.55 ± 8.49 * |
| Δ6–Δ5 | 7.77 ± 7.78 | 7.92 ± 6.30 |
| Variable | Normal-Weight (n = 33) | Overweight/Obese (n = 63) | p-Value |
|---|---|---|---|
| 1. pH before RM | 7.37 ± 0.04 | 7.39 ± 0.04 | 0.016 |
| 2. pH after RM | 7.36 ± 0.04 | 7.37 ± 0.04 | 0.111 |
| 1. PaO2 before RM | 200.79 ± 45.22 | 151.98 ± 41.84 | <0.001 |
| 2. PaO2 after RM | 212.82 ± 33.67 | 173.19 ± 39.00 | <0.001 |
| 1. P/F ratio before RM | 429.18 ± 100.14 | 330.52 ± 88.62 | <0.001 |
| 2. P/F ratio after RM | 457.88 ± 76.38 | 377.87 ± 83.86 | <0.001 |
| 1. PaCO2 before RM | 39.36 ± 3.19 | 38.83 ± 3.38 | 0.452 |
| 2. PaCO2 after RM | 39.21 ± 3.60 | 39.48 ± 3.38 | 0.723 |
| 1. Lactate before RM | 0.94 ± 0.25 | 0.96 ± 0.22 | 0.629 |
| 2. Lactate after RM | 0.98 ± 0.26 | 1.00 ± 0.24 | 0.707 |
| 1. HCO3− before RM | 22.86 ± 2.21 | 23.94 ± 2.80 | 0.059 |
| 2. HCO3− after RM | 22.42 ± 2.39 | 26.25 ± 24.91 | 0.381 |
| 1. BE before RM | −2.08 ± 2.63 | −1.07 ± 2.78 | 0.088 |
| 2. BE after RM | −3.06 ± 2.89 | −2.09 ± 2.78 | 0.113 |
| 1. A-aDO2 before RM | 84.62 ± 49.28 | 125.64 ± 39.71 | <0.001 |
| 1. A-aDO2 after RM | 71.94 ± 39.27 | 102.58 ± 38.33 | 0.001 |
| 1. paO2/pAO2 before RM | 0.71 ± 0.17 | 0.55 ± 0.14 | <0.001 |
| 2. paO2/pAO2 after RM | 0.75 ± 0.13 | 0.63 ± 0.14 | <0.001 |
| Variable | Mean | Standard Deviation | Standard Error |
|---|---|---|---|
| 1. PaO2 | 168.76 | 48.73 | 4.97 |
| 2. PaO2 | 186.81 * | 41.62 | 4.25 |
| Time | ± SD | 25th Percentile | 50th Percentile | 75th Percentile |
|---|---|---|---|---|
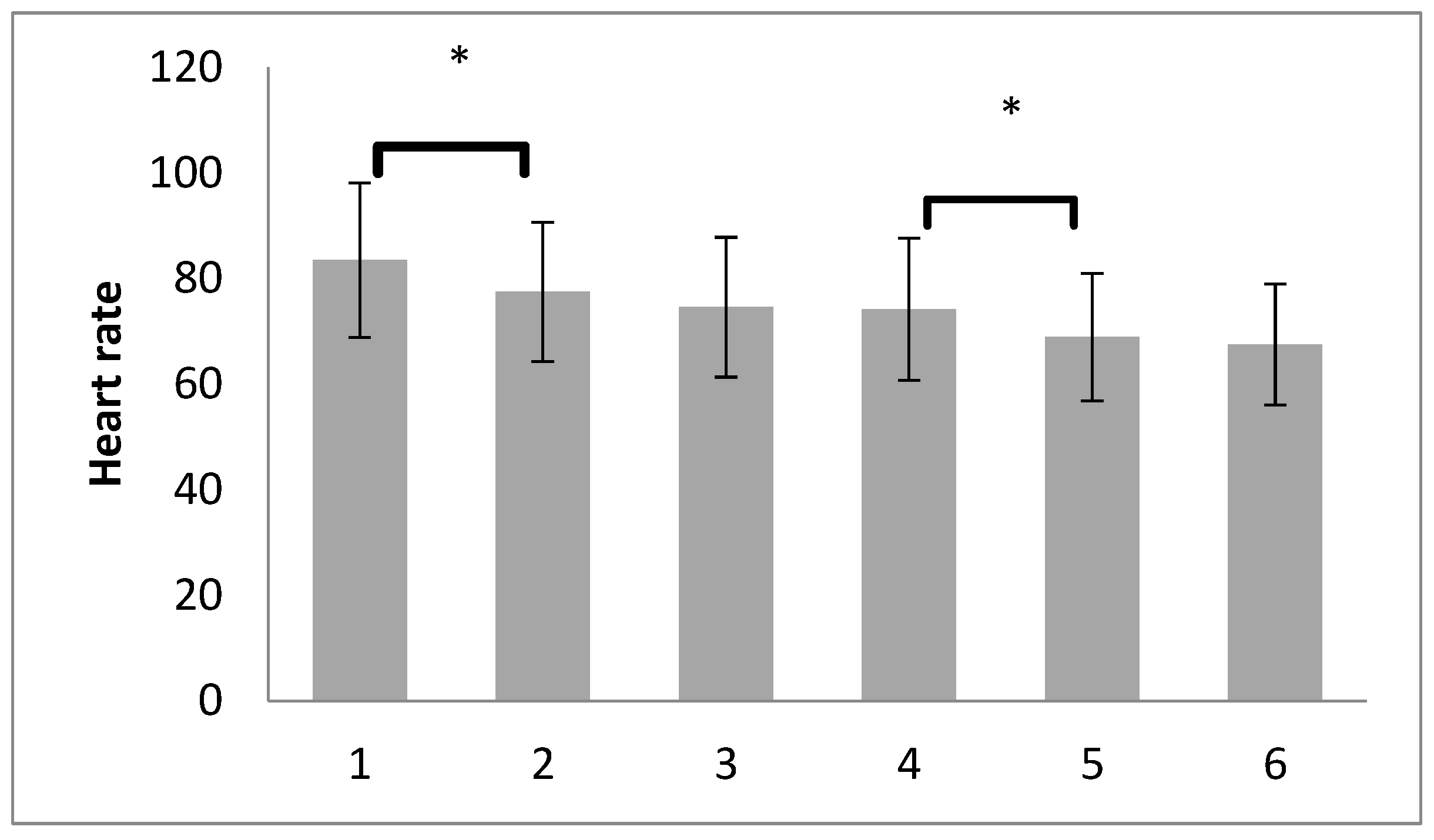
| T1. after induction of anest. | 83.48 ± 14.62 | 72.25 | 81.50 | 93.75 |
| T2. after pneumoperitoneum | 77.46 ± 13.17 | 68.25 | 77.00 | 86.75 |
| T3. after RM with PEEP5 | 74.57 ± 13.24 | 65.00 | 72.00 | 82.75 |
| T4. after RM with PEEP7 | 74.15 ± 13.46 | 64.00 | 72.50 | 83.75 |
| T5. after deflation | 68.90 ± 12.09 | 62.00 | 67.50 | 75.00 |
| T6. after RM at the end | 67.50 ± 11.42 | 59.00 | 66.00 | 75.00 |
| Time | 25th Percentile | 50th Percentile | 75th Percentile | |
|---|---|---|---|---|
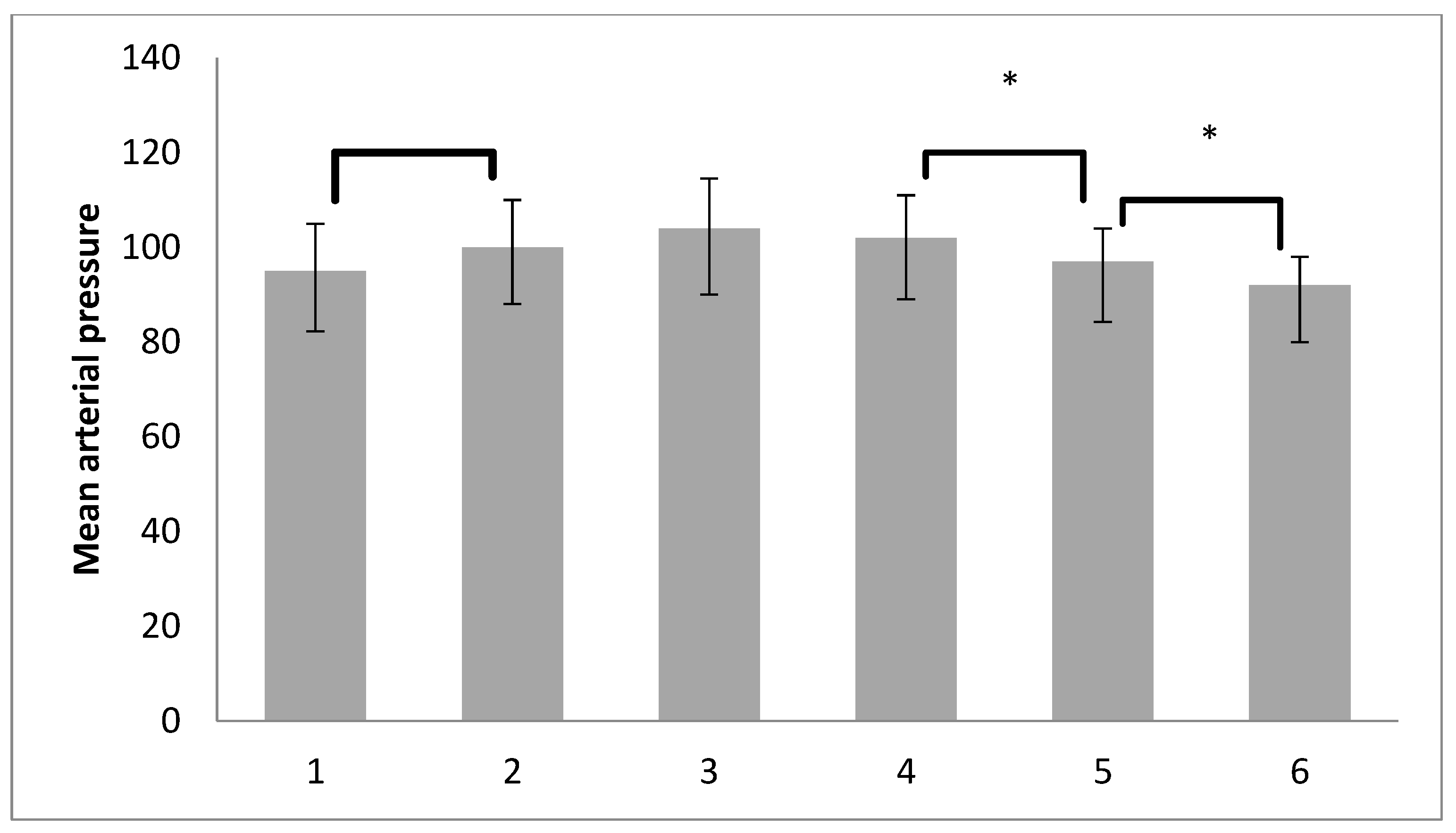
| T1. after induction of anest. | 95.96 ± 14.80 | 85.00 | 95.00 | 107.75 |
| T2. after pneumoperitoneum | 100.89 ± 16.12 | 90.00 | 100.00 | 112.00 |
| T3. after RM with PEEP5 | 105.03 ± 17.59 | 93.50 | 104.00 | 118.00 |
| T4. after RM with PEEP7 | 103.76 ± 15.53 | 93.00 | 102.00 | 115.00 |
| T5. after deflation | 99.29 ± 14.12 | 90.00 | 97.00 | 109.75 |
| T6. after RM at the end | 94.70 ± 13.31 | 86.00 | 92.00 | 104.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anđelić, N.; Uvelin, A.; Stokić, E.; Popović, R.; Zdravković, R.; Preveden, A.; Zornić, N. The Effect of Recruitment Maneuver on Static Lung Compliance in Patients Undergoing General Anesthesia for Laparoscopic Cholecystectomy: A Single-Centre Prospective Clinical Intervention Study. Medicina 2024, 60, 666. https://doi.org/10.3390/medicina60040666
Anđelić N, Uvelin A, Stokić E, Popović R, Zdravković R, Preveden A, Zornić N. The Effect of Recruitment Maneuver on Static Lung Compliance in Patients Undergoing General Anesthesia for Laparoscopic Cholecystectomy: A Single-Centre Prospective Clinical Intervention Study. Medicina. 2024; 60(4):666. https://doi.org/10.3390/medicina60040666
Chicago/Turabian StyleAnđelić, Nada, Arsen Uvelin, Edita Stokić, Radmila Popović, Ranko Zdravković, Andrej Preveden, and Nenad Zornić. 2024. "The Effect of Recruitment Maneuver on Static Lung Compliance in Patients Undergoing General Anesthesia for Laparoscopic Cholecystectomy: A Single-Centre Prospective Clinical Intervention Study" Medicina 60, no. 4: 666. https://doi.org/10.3390/medicina60040666





