Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients
Abstract
:1. Introduction
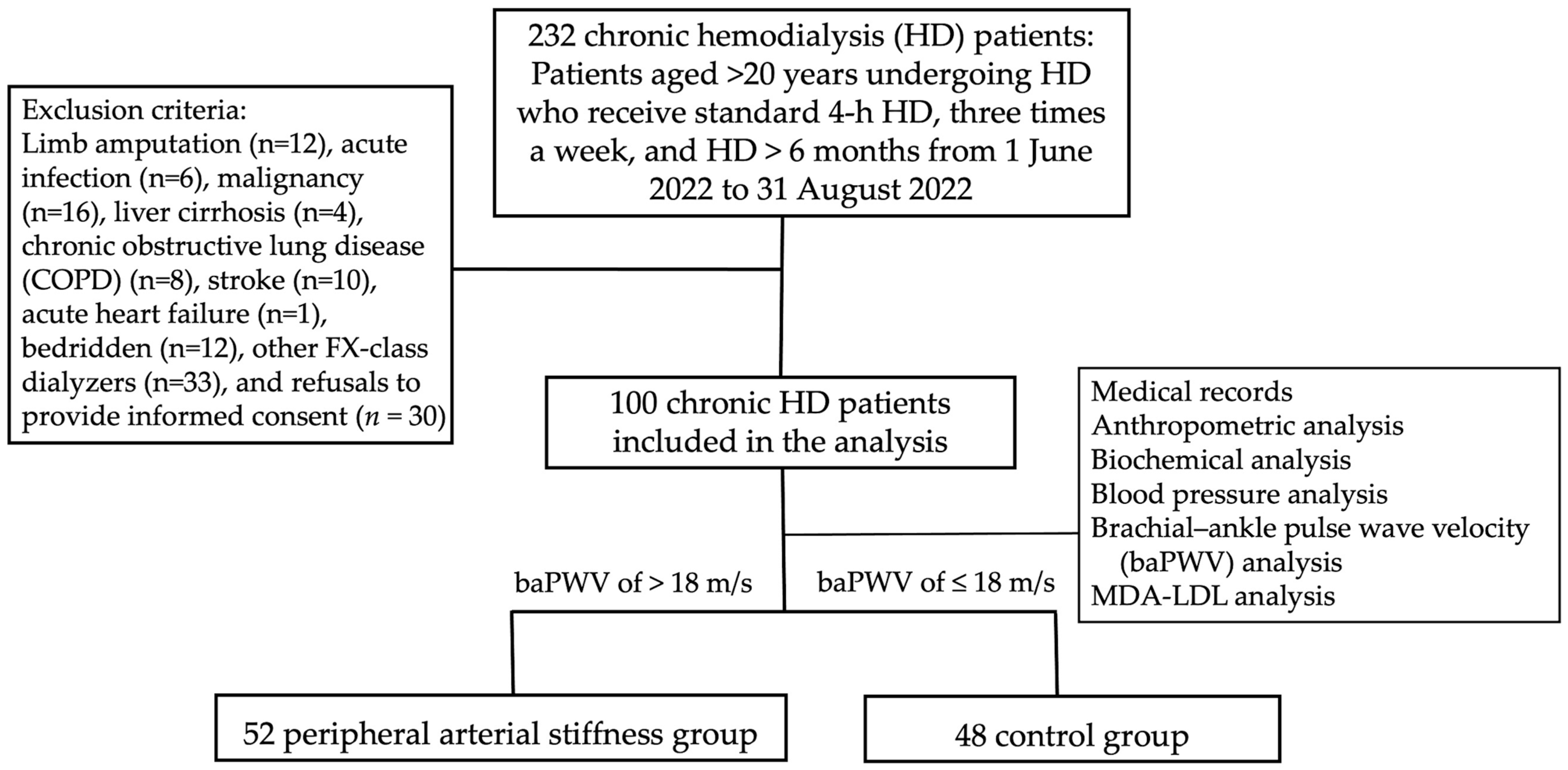
2. Materials and Methods
2.1. Participants and Study Protocol
2.2. Assessment of Anthropometric Parameters
2.3. Laboratory Investigations
2.4. Evaluation of Blood Pressure and Brachial-Ankle Pulse Wave Velocity
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-stage renal disease, inflammation and cardiovascular outcomes. Expand. Hemodial. 2017, 191, 32–43. [Google Scholar]
- Wang, Y.; Gao, L. Inflammation and cardiovascular disease associated with hemodialysis for end-stage renal disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Loutradis, C.; Karpetas, A.; Tzanis, G.; Piperidou, A.; Koutroumpas, G.; Raptis, V.; Syrgkanis, C.; Liakopoulos, V.; Efstratiadis, G. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension 2017, 70, 148–157. [Google Scholar] [CrossRef]
- Matschkal, J.; Mayer, C.C.; Sarafidis, P.A.; Lorenz, G.; Braunisch, M.C.; Guenthner, R.; Angermann, S.; Steubl, D.; Kemmner, S.; Bachmann, Q. Comparison of 24-hour and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am. J. Nephrol. 2019, 49, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef]
- Chen, S.-C.; Huang, J.-C.; Su, H.-M.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Prognostic cardiovascular markers in chronic kidney disease. Kidney Blood Press. Res. 2018, 43, 1388–1407. [Google Scholar] [CrossRef]
- Tsai, J.-P.; Hsu, B.-G. Arterial stiffness: A brief review. Tzu Chi Med. J. 2021, 33, 115–121. [Google Scholar]
- Wung, C.-H.; Wang, Y.-H.; Lee, Y.-C.; Chang, C.-W.; Wu, P.-Y.; Huang, J.-C.; Tsai, Y.-C.; Chen, S.-C.; Chang, J.-M.; Hwang, S.-J. Association between flow-mediated dilation and skin perfusion pressure with peripheral artery disease in hemodialysis patients. J. Pers. Med. 2021, 11, 1251. [Google Scholar] [CrossRef]
- Baba, M.; Maris, M.; Jianu, D.; Luca, C.T.; Stoian, D.; Mozos, I. The impact of the blood lipids levels on arterial stiffness. J. Cardiovasc. Dev. Dis. 2023, 10, 127. [Google Scholar] [CrossRef]
- Wu, C.-F.; Liu, P.-Y.; Wu, T.-J.; Hung, Y.; Yang, S.-P.; Lin, G.-M. Therapeutic modification of arterial stiffness: An update and comprehensive review. World J. Cardiol. 2015, 7, 742. [Google Scholar] [CrossRef]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid peroxidation in atherosclerotic cardiovascular diseases. Antioxid. Redox Signal. 2021, 34, 49–98. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Ito, H. Increased circulating malondialdehyde-modified low-density lipoprotein level is associated with high-risk plaque in coronary computed tomography angiography in patients receiving statin therapy. J. Clin. Med. 2021, 10, 1480. [Google Scholar] [CrossRef]
- Prasad, A.; Clopton, P.; Ayers, C.; Khera, A.; De Lemos, J.A.; Witztum, J.L.; Tsimikas, S. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1213–1221. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis; Endotext: Dartmouth, MA, USA, 2019. [Google Scholar]
- Hou, J.-S.; Wang, C.-H.; Lai, Y.-H.; Kuo, C.-H.; Lin, Y.-L.; Hsu, B.-G.; Tsai, J.-P. Serum malondialdehyde-modified low-density lipoprotein is a risk factor for central arterial stiffness in maintenance hemodialysis patients. Nutrients 2020, 12, 2160. [Google Scholar] [CrossRef]
- Mozos, I.; Luca, C.T. Crosstalk between oxidative and nitrosative stress and arterial stiffness. Curr. Vasc. Pharmacol. 2017, 15, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-G.; Wang, C.-H.; Lin, Y.-L.; Lai, Y.-H.; Tsai, J.-P. Serum trimethylamine N-oxide level is associated with peripheral arterial stiffness in advanced non-dialysis chronic kidney disease patients. Toxins 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Shiina, K. State of the art review: Brachial-ankle PWV. J. Atheroscler. Thromb. 2020, 27, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef]
- Takamura, T.-A.; Tsuchiya, T.; Oda, M.; Watanabe, M.; Saito, R.; Sato-Ishida, R.; Akao, H.; Kawai, Y.; Kitayama, M.; Kajinami, K. Circulating malondialdehyde-modified low-density lipoprotein (MDA-LDL) as a novel predictor of clinical outcome after endovascular therapy in patients with peripheral artery disease (PAD). Atherosclerosis 2017, 263, 192–197. [Google Scholar] [CrossRef]
- Piqueras, L.; Sanz, M.-J. Angiotensin II and leukocyte trafficking: New insights for an old vascular mediator. Role of redox-signaling pathways. Free. Radic. Biol. Med. 2020, 157, 38–54. [Google Scholar] [CrossRef]
- Suciu, C.F.; Prete, M.; Ruscitti, P.; Favoino, E.; Giacomelli, R.; Perosa, F. Oxidized low density lipoproteins: The bridge between atherosclerosis and autoimmunity. Possible implications in accelerated atherosclerosis and for immune intervention in autoimmune rheumatic disorders. Autoimmun. Rev. 2018, 17, 366–375. [Google Scholar] [CrossRef]
- Amirfakhryan, H. Vaccination against atherosclerosis: An overview. Hell. J. Cardiol. 2020, 61, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: A process related to accelerated senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Hsu, S.H.-J.; Wu, Y.-J.; Su, T.-C. Additive effects of postchallenge hyperglycemia and low-density lipoprotein particles on the risk of arterial stiffness in healthy adults. Lipids Health Dis. 2014, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Bobryshev, Y.V.; Sobenin, I.A.; Melnichenko, A.A.; Chistiakov, D.A. Modified low density lipoprotein and lipoprotein-containing circulating immune complexes as diagnostic and prognostic biomarkers of atherosclerosis and type 1 diabetes macrovascular disease. Int. J. Mol. Sci. 2014, 15, 12807–12841. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Maeda, S.; Mukai, J.; Ohki, M.; Nakanishi, M.; Yoshikawa, T. Association between plasma sLOX-1 concentration and arterial stiffness in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 151–155. [Google Scholar] [CrossRef]
- Matsui, S.; Kajikawa, M.; Hida, E.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Oda, N.; Kishimoto, S.; Hidaka, T.; Kihara, Y. Optimal target level of low-density lipoprotein cholesterol for vascular function in statin naïve individuals. Sci. Rep. 2017, 7, 8422. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, H.-K.; Wu, X.-W.; Cao, Z.; Tu, Y.-C.; Ma, Y.; Wang, W.-Q.; Cheng, J.; Zhou, Z.-H. Small dense low density lipoprotein-cholesterol and cholesterol ratios to predict arterial stiffness progression in normotensive subjects over a 5-year period. Lipids Health Dis. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takaeko, Y.; Kajikawa, M.; Kishimoto, S.; Yamaji, T.; Harada, T.; Han, Y.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C. Low levels of low-density lipoprotein cholesterol and endothelial function in subjects without lipid-lowering therapy. J. Clin. Med. 2020, 9, 3796. [Google Scholar] [CrossRef]
- Scarpioni, R.; Ricardi, M.; Melfa, L.; Cristinelli, L. Dyslipidemia in chronic kidney disease: Are statins still indicated in reduction cardiovascular risk in patients on dialysis treatment? Cardiovasc. Ther. 2010, 28, 361–368. [Google Scholar] [CrossRef]
- Moradi, H.; Streja, E.; Vaziri, N.D. ESRD-induced dyslipidemia—Should management of lipid disorders differ in dialysis patients? In Seminars in Dialysis; Whiley: Hoboken, NJ, USA, 2018; pp. 398–405. [Google Scholar]
- Zanoli, L.; Lentini, P.; Briet, M.; Castellino, P.; House, A.A.; London, G.M.; Malatino, L.; McCullough, P.A.; Mikhailidis, D.P.; Boutouyrie, P. Arterial stiffness in the heart disease of CKD. J. Am. Soc. Nephrol. 2019, 30, 918–928. [Google Scholar] [CrossRef]
- An, W.S.; Kim, S.-E.; Kim, K.-H.; Bae, H.-R.; Rha, S.-H. Associations between oxidized LDL to LDL ratio, HDL and vascular calcification in the feet of hemodialysis patients. J. Korean Med. Sci. 2009, 24, S115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Wu, H. Arterial stiffness: A focus on vascular calcification and its link to bone mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Arterial stiffness as a risk factor for clinical hypertension. Nat. Rev. Cardiol. 2018, 15, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mishra, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 001–011. [Google Scholar]
- Lin, G.-M.; Wu, C.-F.; Liu, P.-Y.; Han, C.-L. Modified low-density lipoprotein may moderate the association of baseline hs-CRP with incident cardiac events in the Asian populations. J. Cardiol. 2016, 68, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-M.; Liu, K.; Colangelo, L.A.; Lakoski, S.G.; Tracy, R.P.; Greenland, P. Low-density lipoprotein cholesterol concentrations and association of high-sensitivity C-reactive protein concentrations with incident coronary heart disease in the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2016, 183, 46–52. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 2017, 174, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual cardiovascular risk at low LDL: Remnants, lipoprotein (a), and inflammation. Clin. Chem. 2021, 67, 143–153. [Google Scholar] [CrossRef]
- Aminuddin, A.; Lazim, M.R.M.; Hamid, A.A.; Hui, C.K.; Mohd Yunus, M.H.; Kumar, J.; Ugusman, A. The association between inflammation and pulse wave velocity in dyslipidemia: An evidence-based review. Mediat. Inflamm. 2020, 2020, 4732987. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and metabolic protection by vitamin E: A matter of treatment strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.D.; Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016, 15, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Sprick, J.D.; Mammino, K.; Jeong, J.; DaCosta, D.R.; Hu, Y.; Morison, D.G.; Nocera, J.R.; Park, J. Aerobic exercise training improves endothelial function and attenuates blood pressure reactivity during maximal exercise in chronic kidney disease. J. Appl. Physiol. 2022, 132, 758–793. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health: A critical review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
| Items | All Participants (n = 100) | baPWV ≤ 18 m/s Group (n = 48) | baPWV > 18 m/s Group (n = 52) | p Value |
|---|---|---|---|---|
| Age (years) | 63.84 ± 13.51 | 57.94 ± 13.35 | 67.37 ± 12.11 | <0.001 * |
| HD duration (months) | 55.92 (21.96–123.60) | 55.44 (19.44–132.00) | 56.16 (24.42–112.62) | 0.992 |
| Height (cm) | 160.70 ± 7.43 | 162.09 ± 9.19 | 159.47 ± 5.21 | 0.079 |
| Pre-HD body weight (kg) | 63.52 ± 15.13 | 66.37 ± 16.16 | 60.89 ± 13.74 | 0.070 |
| Post-HD body weight (kg) | 61.36 ± 14.68 | 64.05 ± 15.76 | 58.88 ± 13.29 | 0.079 |
| Body mass index (kg/m2) | 24.49 ± 5.20 | 25.16 ± 5.73 | 23.90 ± 4.66 | 0.226 |
| Waist circumference (cm) | 90.68 ± 12.48 | 90.40 ± 13.32 | 90.94 ± 11.76 | 0.828 |
| Systolic blood pressure (mmHg) | 140.49 ± 25.73 | 138.31 ± 24.81 | 142.50 ± 26.64 | 0.419 |
| Diastolic blood pressure (mmHg) | 76.60 ± 15.16 | 78.50 ± 15.33 | 74.85 ± 14.93 | 0.230 |
| Left baPWV (m/s) | 18.04 ± 3.25 | 15.47 ± 1.91 | 20.41 ± 2.28 | <0.001 * |
| Right baPWV (m/s) | 18.14 ± 3.29 | 15.38 ± 1.55 | 20.68 ± 2.26 | <0.001 * |
| Hemoglobin (g/dL) | 10.41 ± 1.18 | 10.36 ± 1.26 | 10.46 ± 1.11 | 0.652 |
| Albumin (g/dL) | 4.15 ± 0.43 | 4.25 ± 0.41 | 4.06 ± 0.43 | 0.022 * |
| Total cholesterol (mg/dL) | 149.14 ± 35.87 | 145.88 ± 33.97 | 152.15 ± 37.61 | 0.384 |
| Triglyceride (mg/dL) | 153.91 ± 80.23 | 152.54 ± 75.19 | 155.17 ± 85.33 | 0.871 |
| MDA-LDL (mg/dL) | 88.67 (69.96–148.52) | 78.38 (59.41–98.35) | 119.67 (81.65–176.54) | <0.001 * |
| Glucose (mg/dL) | 132.50 (110.00–171.25) | 131.00 (104.00–172.00) | 133.00 (113.00–173.00) | 0.377 |
| Blood urea nitrogen (mg/dL) | 59.18 ± 14.21 | 61.71 ± 14.68 | 56.85 ± 13.49 | 0.087 |
| Creatinine (mg/dL) | 9.41 ± 1.87 | 9.85 ± 1.98 | 8.99 ± 1.67 | 0.021 * |
| Total calcium (mg/dL) | 8.90 ± 0.75 | 8.86 ± 0.67 | 8.95 ± 0.83 | 0.549 |
| Phosphorus (mg/dL) | 4.55 ± 1.25 | 4.68 ± 1.28 | 4.43 ± 1.22 | 0.326 |
| Intact parathyroid hormone (pg/mL) | 204.05 (56.83–355.30) | 205.20 (69.40–461.30) | 192.90 (54.90–340.65) | 0.297 |
| C-reactive protein (mg/dL) | 0.28 (0.06–0.66) | 0.16 (0.05–0.40) | 0.35 (0.10–1.01) | 0.007 * |
| Urea reduction rate | 0.74 ± 0.05 | 0.73 ± 0.05 | 0.74 ± 0.04 | 0.181 |
| Kt/V (Gotch) | 1.36 ± 0.19 | 1.33 ± 0.21 | 1.38 ± 0.18 | 0.195 |
| Female, n (%) | 47 (47.0) | 18 (38.3) | 29 (54.7) | 0.101 |
| Diabetes mellitus, n (%) | 46 (46.0) | 19 (40.4) | 27 (50.9) | 0.292 |
| Hypertension, n (%) | 46 (46.0) | 22 (46.8) | 24 (45.3) | 0.879 |
| Angiotensin receptor blocker, n (%) | 28 (28.0) | 15 (31.9) | 13 (24.5) | 0.412 |
| β-blocker, n (%) | 35 (35.0) | 15 (31.9) | 20 (37.7) | 0.542 |
| Calcium channel blocker, n (%) | 40 (40.0) | 21 (44.7) | 19 (35.8) | 0.368 |
| Statin, n (%) | 31 (31.0) | 13 (27.7) | 18 (34.0) | 0.496 |
| Fibrate, n (%) | 25 (25.0) | 12 (25.5) | 13 (24.5) | 0.908 |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| MDA-LDL, 1 mg/mL | 1.014 | 1.004–1.025 | 0.009 * |
| Age, 1 year | 1.044 | 1.004–1.085 | 0.031 * |
| C-reactive protein, 1 mg/dL | 3.697 | 1.149–11.893 | 0.028 * |
| Albumin, 1 g/dL | 0.526 | 0.144–1.926 | 0.332 |
| Creatinine, 1 mg/dL | 0.971 | 0.734–1.285 | 0.837 |
| Variables | Left baPWV (m/s) | Right baPWV (m/s) | ||
|---|---|---|---|---|
| Spearman’s Coefficient of Correlation | p Value | Spearman’s Coefficient of Correlation | p Value | |
| Age (years) | 0.284 | 0.004 * | 0.308 | 0.002 * |
| Log-HD duration (months) | –0.037 | 0.711 | 0.009 | 0.930 |
| Height (cm) | –0.115 | 0.256 | –0.136 | 0.178 |
| Pre-HD body weight (kg) | –0.168 | 0.094 | –0.147 | 0.146 |
| Body mass index (kg/m2) | –0.146 | 0.146 | –0.115 | 0.253 |
| Waist circumference (cm) | 0.003 | 0.973 | 0.029 | 0.778 |
| Systolic blood pressure (mmHg) | 0.121 | 0.232 | 0.119 | 0.238 |
| Diastolic blood pressure (mmHg) | –0.070 | 0.487 | –0.096 | 0.343 |
| Left baPWV (m/s) | — | — | 0.875 | <0.001 * |
| Right baPWV (m/s) | 0.875 | <0.001 * | — | — |
| Hemoglobin (g/dL) | 0.053 | 0.601 | 0.054 | 0.593 |
| Albumin (g/dL) | –0.160 | 0.111 | –0.180 | 0.072 |
| Total cholesterol (mg/dL) | 0.025 | 0.806 | 0.060 | 0.553 |
| Triglyceride (mg/dL) | 0.075 | 0.461 | 0.021 | 0.835 |
| Log-MDA-LDL (mg/dL) | 0.385 | <0.001 * | 0.390 | <0.001 * |
| Log-Glucose (mg/dL) | 0.227 | 0.023 * | 0.191 | 0.054 |
| Blood urea nitrogen (mg/dL) | –0.126 | 0.212 | –0.101 | 0.317 |
| Creatinine (mg/dL) | –0.221 | 0.027 * | –0.216 | 0.031 * |
| Total calcium (mg/dL) | –0.100 | 0.323 | –0.031 | 0.758 |
| Phosphorus (mg/dL) | –0.099 | 0.329 | –0.070 | 0.466 |
| Log-iPTH (pg/mL) | –0.184 | 0.067 | –0.085 | 0.403 |
| Log-CRP (mg/dL) | 0.307 | 0.002 * | 0.249 | 0.013 * |
| Urea reduction rate | 0.184 | 0.067 | 0.106 | 0.292 |
| Kt/V (Gotch) | 0.195 | 0.052 | 0.154 | 0.127 |
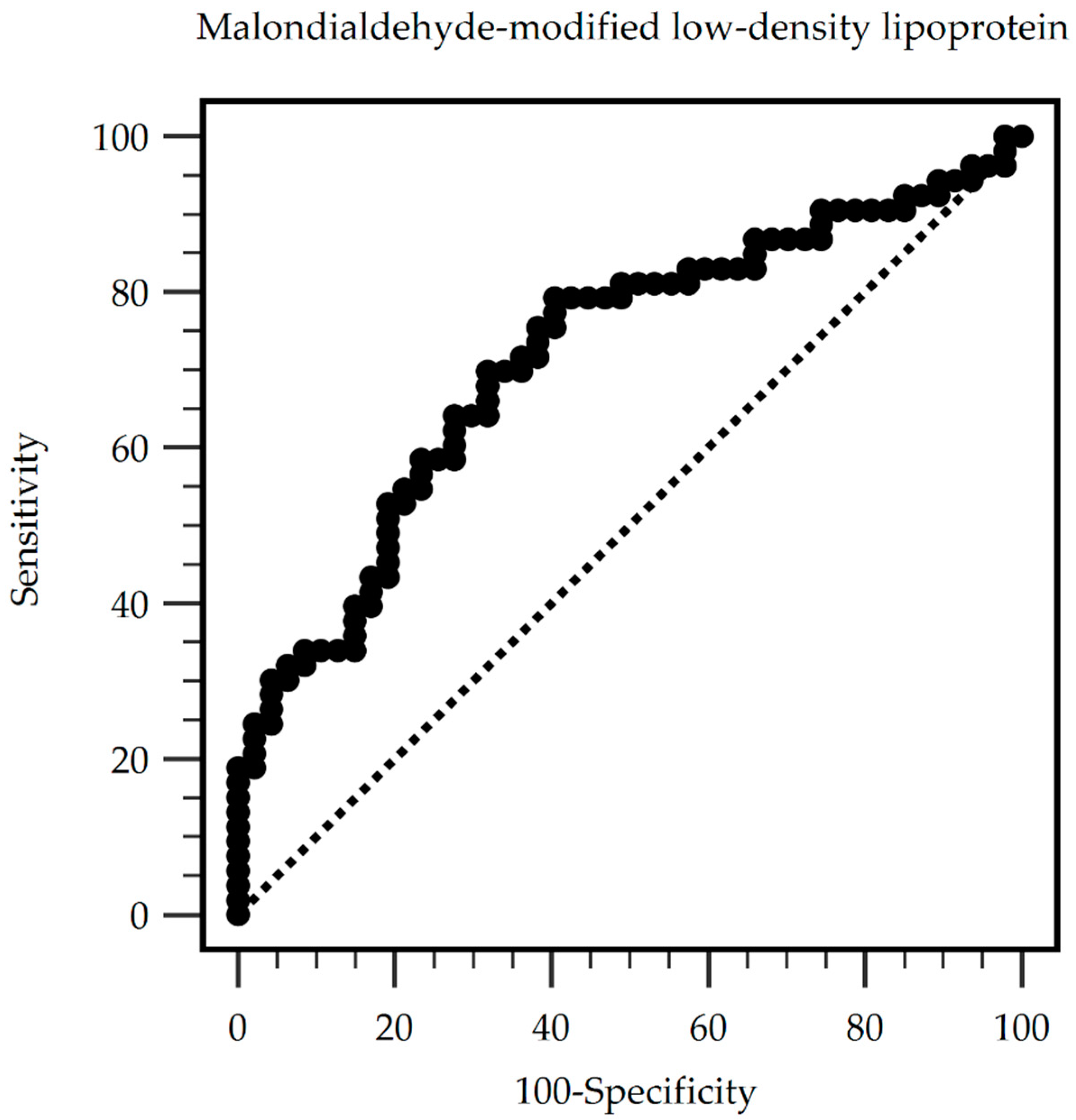
| Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 80.91 mg/dL | 79.25% | 59.57% | 68.85% | 71.80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-N.; Hsu, Y.-C.; Lu, C.-W.; Lin, S.-C.; Wu, T.-J.; Lin, G.-M. Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients. Medicina 2024, 60, 697. https://doi.org/10.3390/medicina60050697
Liu W-N, Hsu Y-C, Lu C-W, Lin S-C, Wu T-J, Lin G-M. Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients. Medicina. 2024; 60(5):697. https://doi.org/10.3390/medicina60050697
Chicago/Turabian StyleLiu, Wei-Nung, Yi-Chiung Hsu, Chia-Wen Lu, Ssu-Chin Lin, Tsung-Jui Wu, and Gen-Min Lin. 2024. "Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients" Medicina 60, no. 5: 697. https://doi.org/10.3390/medicina60050697






