Gene Cloning, Expression and Characterization of a Novel Xylanase from the Marine Bacterium, Glaciecola mesophila KMM241
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids and Chemicals
2.2. Gene Cloning of xynB
2.3. Expression and Purification of XynB
2.4. Enzyme Assay and Protein Determination
2.5. Enzyme Characterization
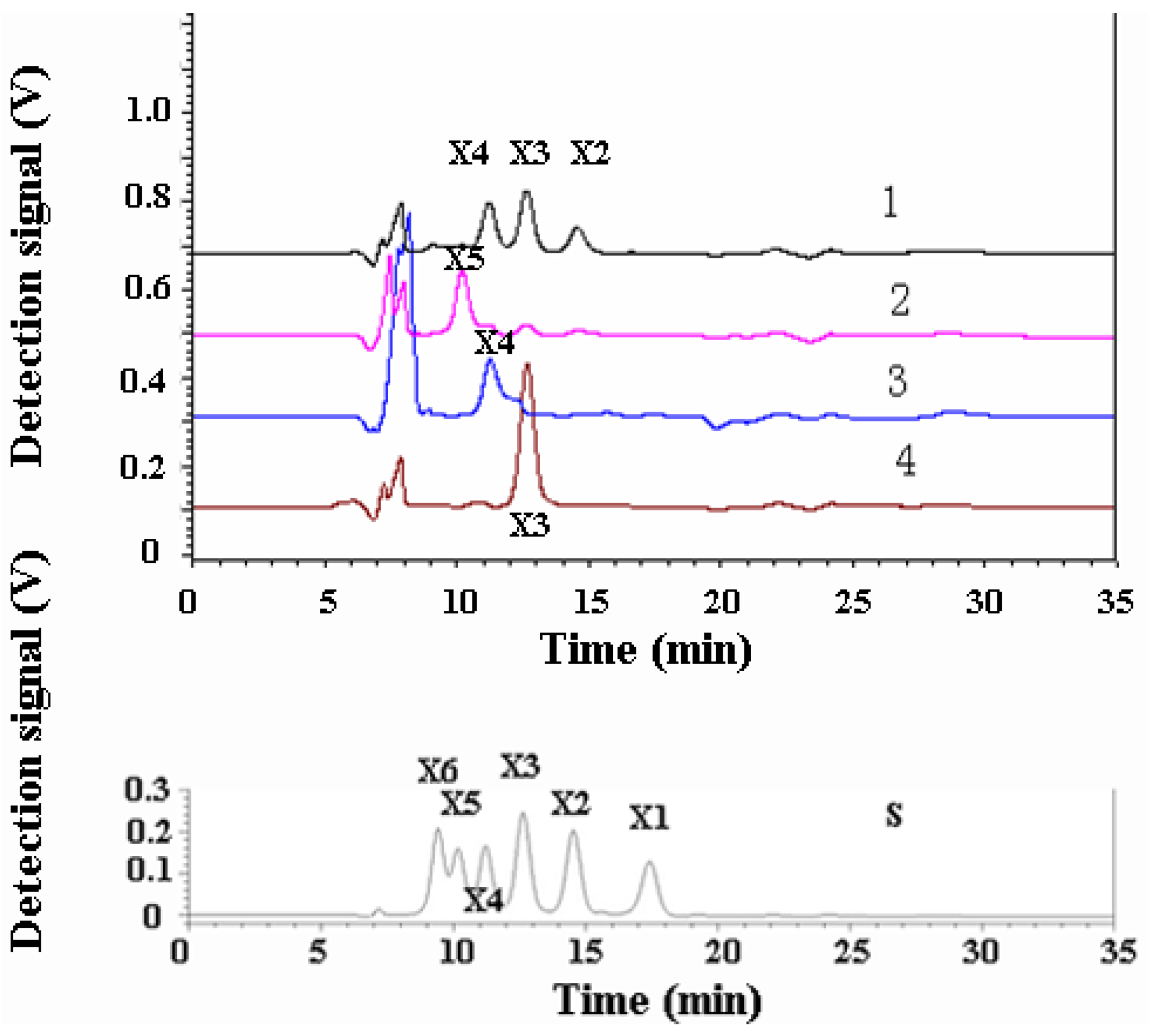
2.6. Analysis of the Products of Xylo-Oligosaccharides Hydrolyzed by XynB
2.7. Expression and Purification of the N-Terminal Domain
2.8. Insoluble Polysaccharide-Binding Assay of the N-Terminal Domain
3. Results
3.1. Gene Cloning and Sequence Analysis
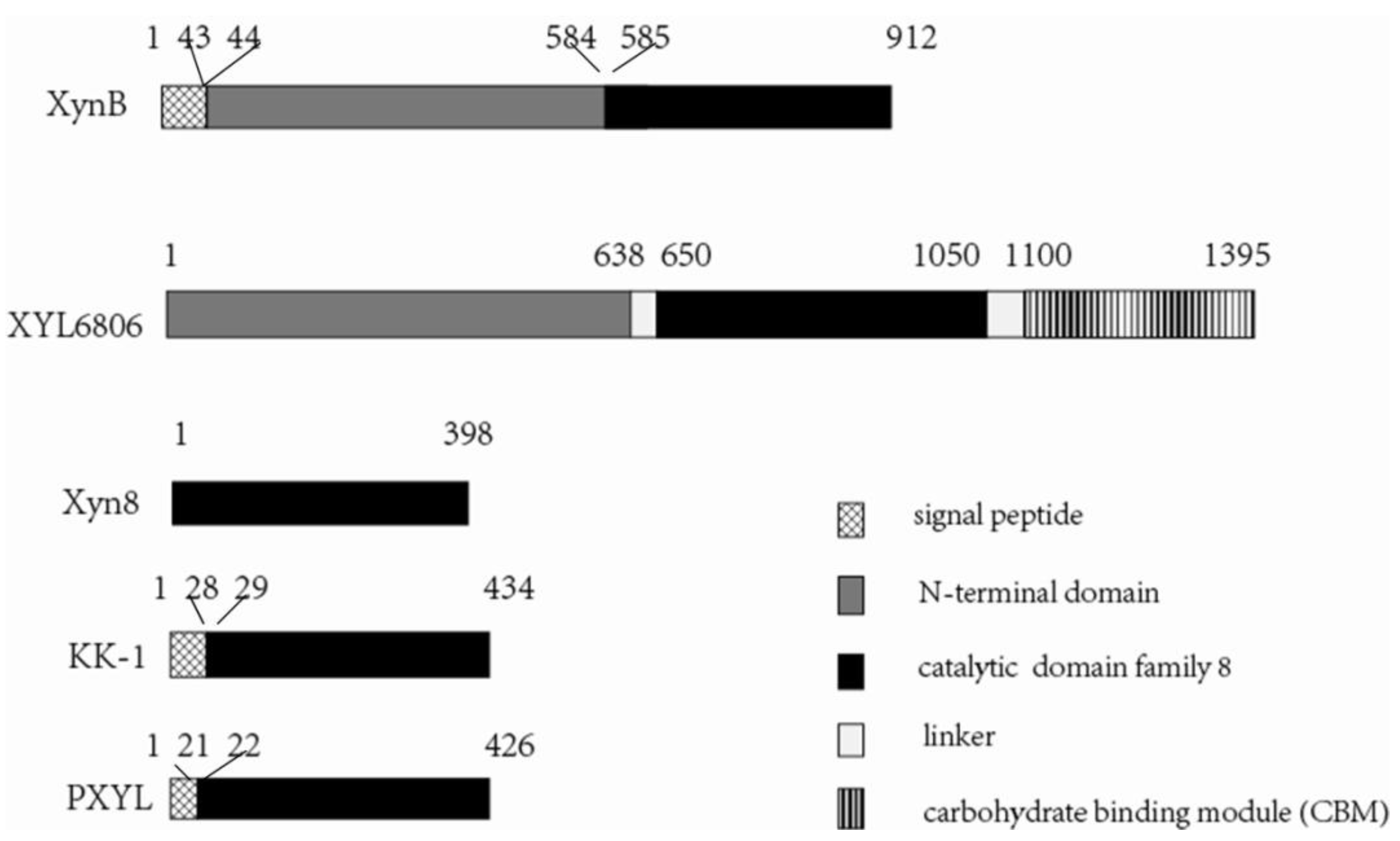
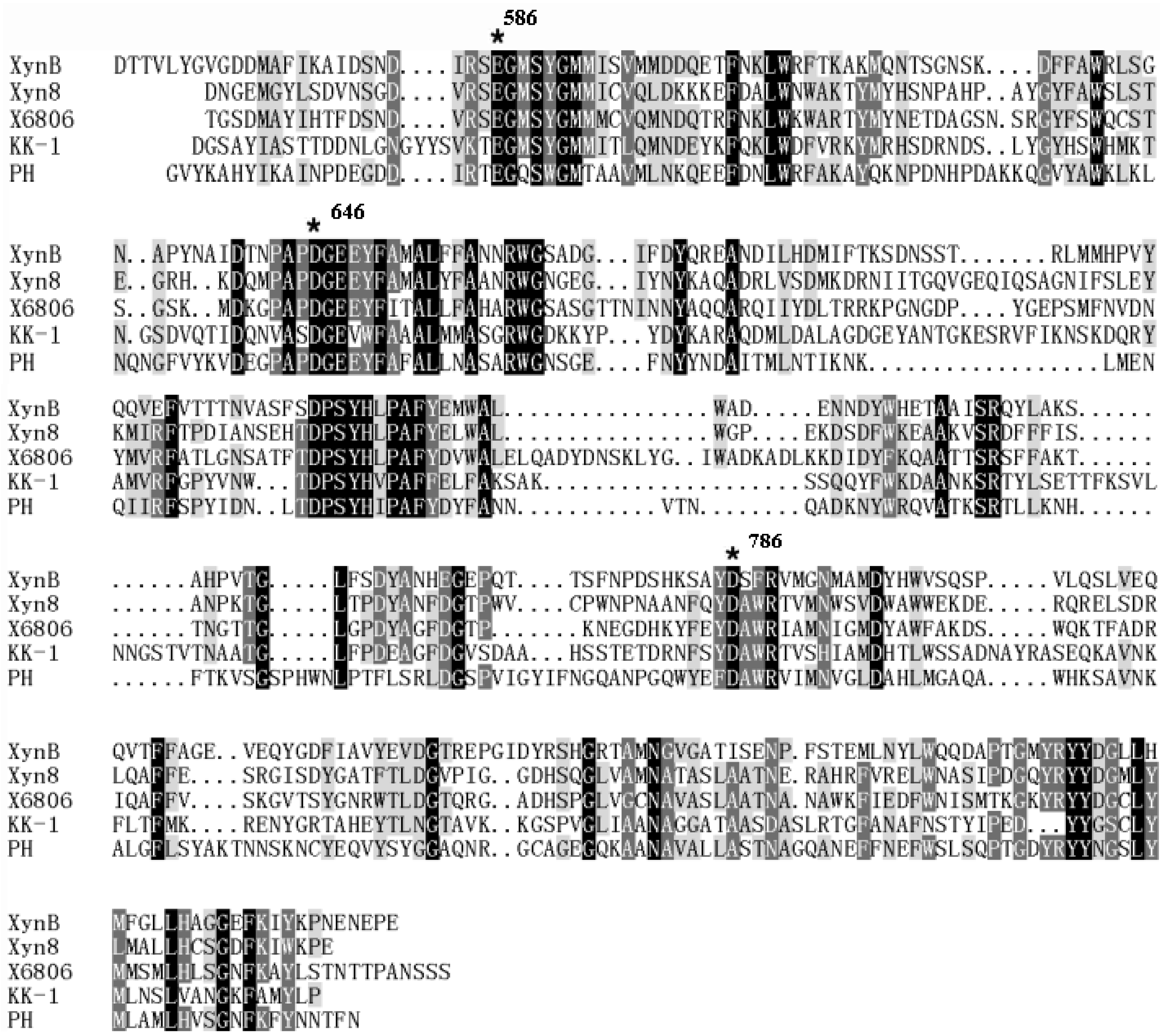
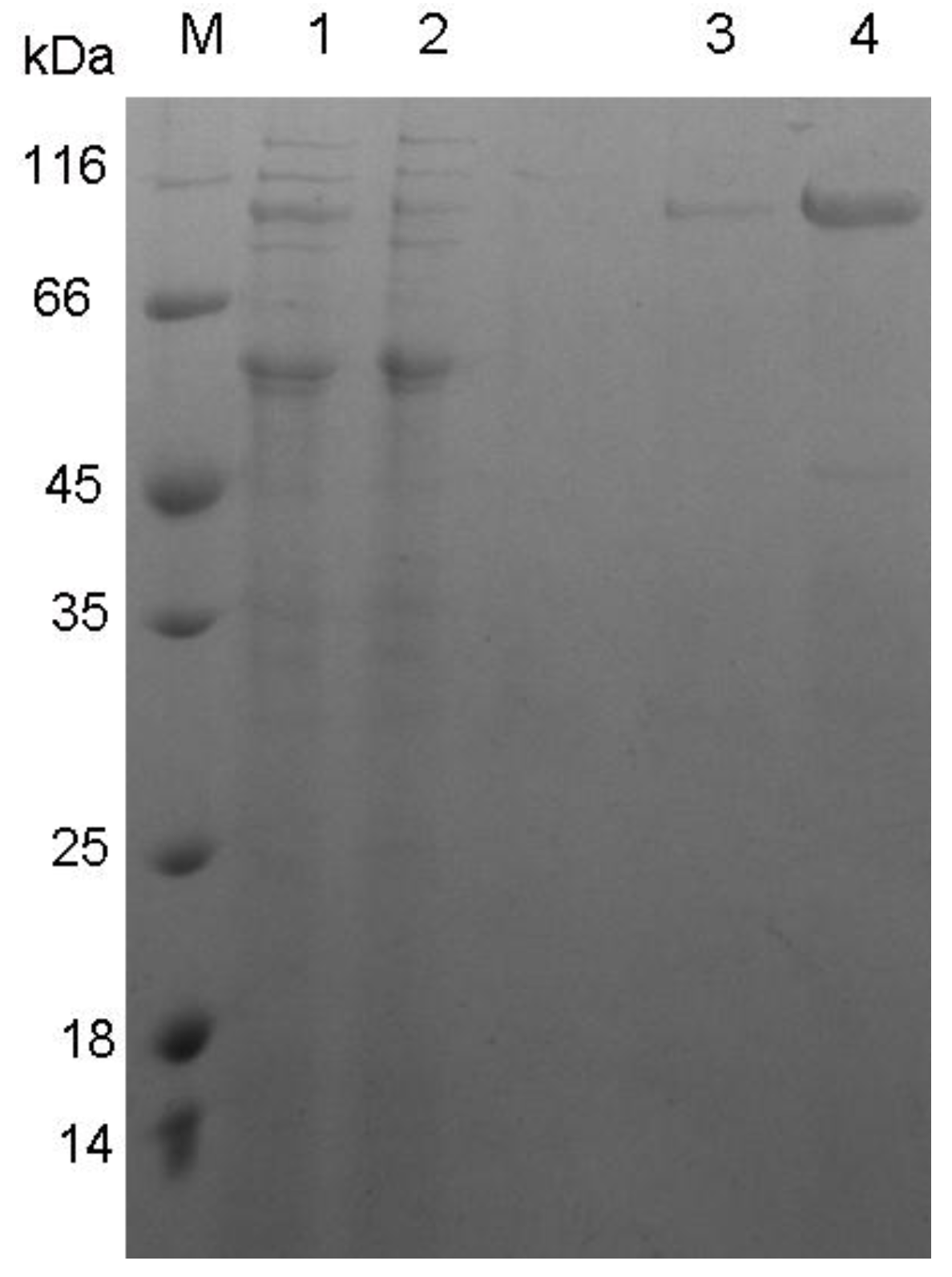
3.2. Expression and Characterization of XynB

| Metal ions and chemical reagents | Relative activity (%) | |
|---|---|---|
| 1 mM | 5 mM | |
| control | 100 ± 1.2 | 100 ± 0.5 |
| Urea | 99.2 ± 3.1 | 101.5 ± 2.4 |
| Sn2+ | 96.3 ± 0.7 | 100.4 ± 1.7 |
| Ca2+ | 97.9 ± 0.8 | 95.6 ± 1.1 |
| K+ | 101.3 ± 2.1 | 93.7 ± 0.8 |
| Sr2+ | 95.7 ± 1.3 | 94.5 ± 2.3 |
| Mg2+ | 98.7 ± 0.3 | 89.7 ± 1.9 |
| Fe3+ | 92.5 ± 0.5 | 87.7 ± 3.6 |
| Cu2+ | 95.7 ± 1.9 | 86.4 ± 2.7 |
| Li2+ | 94.0 ± 0.8 | 76.2 ± 0.8 |
| EDTA | 85.8 ± 2.1 | 67.6 ± 1.4 |
| SDS | 84.9 ± 1.0 | 65.0 ± 1.8 |
| Mn2+ | 73.4 ± 2.2 | 54.2 ± 0.6 |
| Ni2+ | 90.7 ± 3.8 | 49.7 ± 0.9 |
| Zn2+ | 81.1 ± 0.7 | 39.0 ± 2.5 |
| Co2+ | 64.2 ± 0.6 | 8.5 ± 2.2 |

| Xylan | Km (mg/mL) | Vmax (mmol/min·mg) | kcat (1/s) | kcat/Km (mL/mg·s) |
|---|---|---|---|---|
| Beech wood xylan | 5.82 | 0.38 | 609 | 104.64 |
| Oat spelt xylan | 11.86 | 0.44 | 712 | 60.03 |
3.3. Hydrolysis Product Analysis
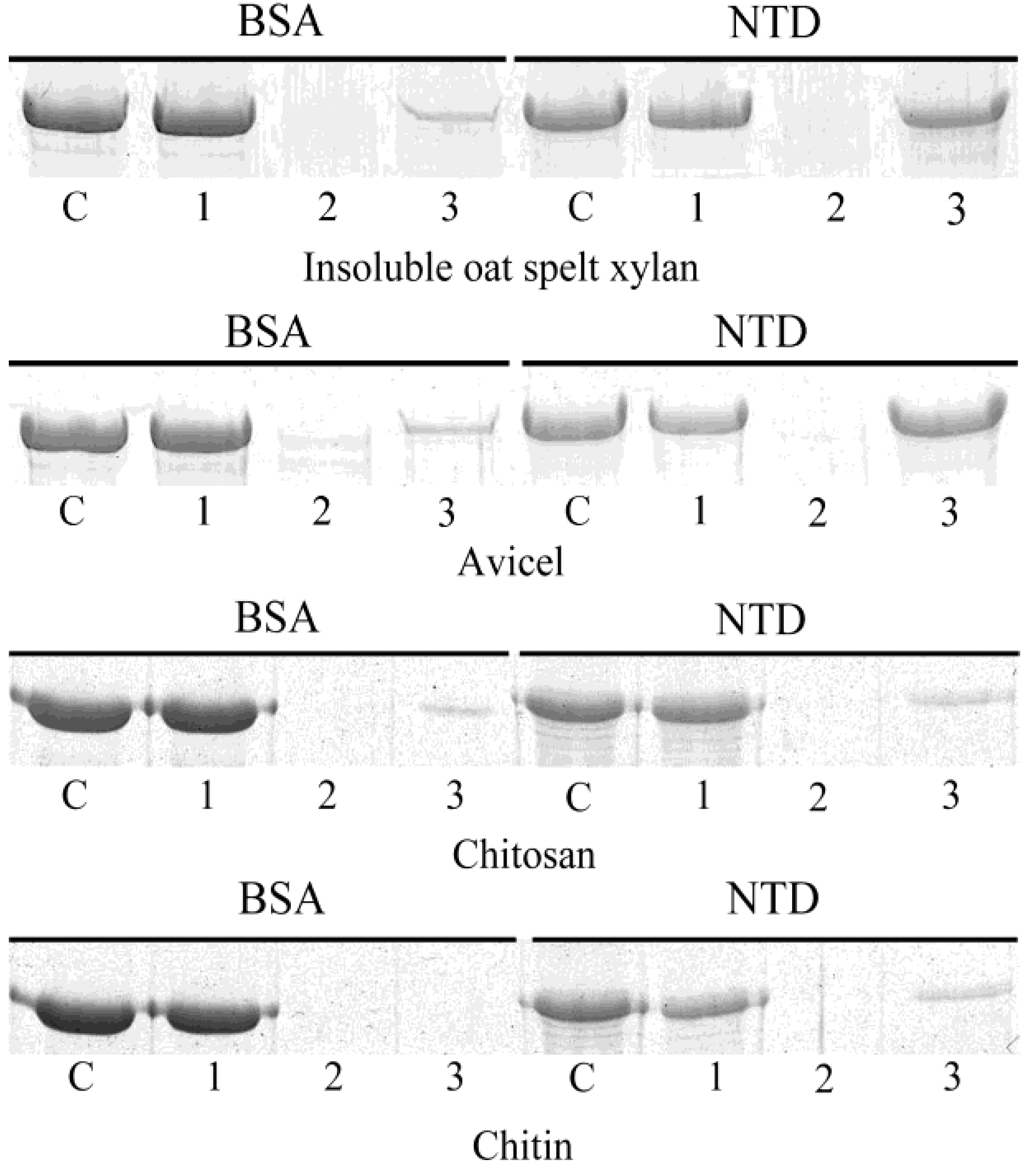
3.4. Insoluble Polysaccharide-Binding Ability of the N-Terminal Domain
4. Discussion
5. Conclusion
Acknowledgments
References
- Beg, Q.K.; Kapoor, M.; Mahajan, L.; Hoondal, G.S. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001, 56, 326–338. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Enzymology of Xylan Degradation. In Biopolymers; Imam, S.H., Greene, R.V., Zaidi, B.R., Eds.; American Chemical Society: Washington, DC, USA, 1999; pp. 167–194. [Google Scholar]
- Dhiman, S.S.; Sharma, J.; Battana, B. Industrial applications and future prospects of microbial xylanases: A review. BioResources 2008, 3, 1377–1402. [Google Scholar]
- Juturu, V.; Wu, J.C. Microbial xylanases: Engineering, production and industrial applications. Biotechnol. Adv. 2011, 30, 1219–1227. [Google Scholar] [CrossRef]
- Pollet, A.; Schoepe, J.; Dornez, E.; Strelkov, S.V.; Delcour, J.A.; Courtin, C.M. Functional analysis of glycoside hydrolase family 8 xylanases shows narrow but distinct substrate specificities and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 87, 2125–2135. [Google Scholar] [CrossRef]
- Yoon, K.H.; Yun, H.N.; Jung, K.H. Molecular cloning of a Bacillus sp. KK-1 xylanase gene and characterization of the gene product. Biochem. Mol. Biol. Int. 1998, 45, 337–347. [Google Scholar]
- Collins, T.; Meuwis, M.A.; Stals, I.; Claeyssens, M.; Feller, G.; Gerday, C. A novel family 8 xylanase: Functional and physicochemical characterization. J. Biol. Chem. 2002, 277, 35133–35139. [Google Scholar]
- Brennan, Y.; Callen, W.N.; Christoffersen, L.; Dupree, P.; Goubet, F.; Healey, S.; Hernandez, M.; Keller, M.; Li, K.; Palackal, N.; et al. Unusual microbial xylanases from insect guts. Appl. Environ. Microbiol. 2004, 70, 3609–3617. [Google Scholar]
- Lee, C.C.; Kibblewhite-Accinelli, R.E.; Wagschal, K.; Robertson, G.H.; Wong, D.W. Cloning and characterization of a cold-active xylanase enzyme from an environmental DNA library. Extremophiles 2006, 10, 295–300. [Google Scholar] [CrossRef]
- Honda, Y.; Kitaoka, M. A family 8 glycoside hydrolase from Bacillus halodurans C-125 (BH2105) is a reducing end xylose-releasing exo-oligoxylanase. J. Biol. Chem. 2004, 279, 55097–55103. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Lloyd, R.M.; Beldman, G.; Verdoes, J.C.; McCleary, B.V.; Voragen, A.G.J. Cloning and characterization of arabinofuranohydrolase-D3 (AXHd3) from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 2005, 67, 641–647. [Google Scholar]
- Wu, S.; Liu, B.; Zhang, X. Characterization of a recombinant thermostable xylanase from deep-sea thermophilic Geobacillus sp. MT-1 in East Pacific. Appl. Microbiol. Biotechnol. 2006, 72, 1210–1216. [Google Scholar] [CrossRef]
- Guo, B.; Chen, X.L.; Sun, C.Y.; Zhou, B.C.; Zhang, Y.Z. Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM241. Appl. Microbiol. Biotechnol. 2009, 84, 1107–1115. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.Q.; Bai, F.W. Production of xylanase by an alkaline-tolerant marine-derived Streptomyces viridochromogenes strain and improvement by ribosome engineering. Appl. Microbiol. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Romanenko, L.A.; Zhukova, N.V.; Rohde, M.; Lysenko, A.M.; Mikhailov, V.V.; Stackebrandt, E. Glaciecola mesophila sp. nov., a novel marine agar-digesting bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 647–651. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, B.; Chen, X.L.; Fan, S.J.; Zhang, Y.Z. Improvement of the quality of wheat bread by addition of glycoside hydrolase family 10 xylanases. Appl. Microbiol. Biotechnol. 2011, 90, 509–515. [Google Scholar] [CrossRef]
- Saito, H.; Miura, K. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim. Biophys. Acta 1963, 72, 619–629. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Miller, G.L.; Blum, R.; Glennon, W.E.; Burton, A.L. Measurement of carboxymethyl cellulase activity. Anal. Biochem. 1960, 2, 127–132. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pason, P.; Kyu, K.L.; Ratanakhanokchai, K. Paenibacillus curdlanolyticus strain B-6 xylanolytic-cellulolytic enzyme system that degrades insoluble polysaccharides. Appl. Environ. Microbiol. 2006, 72, 2483–2490. [Google Scholar] [CrossRef]
- Valenzuela, S.V.; Diaz, P.; Pastor, F.L.J. Modular glucuronoxylan-specific xylanase with a family CBM35 carbohydrate-binding module. Appl. Environ. Microbiol. 2012, 78, 3923–3931. [Google Scholar] [CrossRef]
- Cantarel, B.C.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, T.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Hung, K.S.; Liu, S.M.; Tzou, W.S.; Lin, F.P.; Pan, C.L.; Fang, T.Y.; Sun, K.H.; Tang, S.J. Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Proc. Biochem. 2011, 46, 1257–1263. [Google Scholar] [CrossRef]
- Fialho, M.B.; Carmona, E.C. Purification and characterization of xylanase from Aspergillus giganteus. Folia Microbiol. 2004, 49, 13–18. [Google Scholar] [CrossRef]
- Gupta, S.; Bhushan, B.; Hoondal, G.S. Isolation, purification and characterization of xylanase from Staphylococcus sp. SG-13 and its application in biobleaching of kraft pulp. J. Appl. Microbiol. 2000, 88, 325–334. [Google Scholar] [CrossRef]
- Yamaura, I.; Koga, T.; Matsumoto, T.; Kato, T. Purification and some properties of endo-1,4-β-d-xylanase from a fresh-water mollusc, Pomacea insularus (de Ordigny). Biosci. Biotechnol. Biochem. 1997, 61, 615–620. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, J.; Huang, G.; Ma, L.; Zhang, X. Molecular cloning and heterologous expression of a new xylanase gene from Plectosphaerella cucumerina. Appl. Microbiol. Biotechnol. 2007, 74, 339–346. [Google Scholar] [CrossRef]
- Ali, M.K.; Hayashi, H.; Karita, S.; Goto, M.; Kimura, T.; Sakka, K.; Ohmiya, K. Importance of the carbohydrate-binding module of Clostridium stercorarium Xyn10B to xylan hydrolysis. Biosci. Biotechnol. Biochem. 2001, 65, 41–47. [Google Scholar]
- Carrard, G.; Koivula, A.; Soderlund, H.; Beguin, P. Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc. Natl. Acad. Sci. USA 2000, 97, 10342–10347. [Google Scholar] [CrossRef]
- Sunna, A.; Gibbs, M.D.; Bergquist, P.L. A novel thermostable multidomain 1,4-β-xylanase from ‘Caldibacillus cellulovorans’ and effect of its xylan-binding domain on enzyme activity. Microbiology 2000, 146, 2947–2955. [Google Scholar]
- Waeonukul, R.; Pason, P.; Kyu, K.L.; Sakka, K.; Kosugi, A.; Mori, Y.; Ratanakhanokchai, K. Cloning, sequencing, and expression of the gene encoding a multidomain endo-β-1,4-xylanase from Paenibacillus curdlanolyticus B-6, and characterization of the recombinant enzyme. J. Microbiol. Biotechnol. 2009, 19, 277–285. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, B.; Li, P.-Y.; Yue, Y.-S.; Zhao, H.-L.; Dong, S.; Song, X.-Y.; Sun, C.-Y.; Zhang, W.-X.; Chen, X.-L.; Zhang, X.-Y.; et al. Gene Cloning, Expression and Characterization of a Novel Xylanase from the Marine Bacterium, Glaciecola mesophila KMM241. Mar. Drugs 2013, 11, 1173-1187. https://doi.org/10.3390/md11041173
Guo B, Li P-Y, Yue Y-S, Zhao H-L, Dong S, Song X-Y, Sun C-Y, Zhang W-X, Chen X-L, Zhang X-Y, et al. Gene Cloning, Expression and Characterization of a Novel Xylanase from the Marine Bacterium, Glaciecola mesophila KMM241. Marine Drugs. 2013; 11(4):1173-1187. https://doi.org/10.3390/md11041173
Chicago/Turabian StyleGuo, Bing, Ping-Yi Li, Yong-Sheng Yue, Hui-Lin Zhao, Sheng Dong, Xiao-Yan Song, Cai-Yun Sun, Wei-Xin Zhang, Xiu-Lan Chen, Xi-Ying Zhang, and et al. 2013. "Gene Cloning, Expression and Characterization of a Novel Xylanase from the Marine Bacterium, Glaciecola mesophila KMM241" Marine Drugs 11, no. 4: 1173-1187. https://doi.org/10.3390/md11041173




